Medical chemistry just after the 1970s brought real attention to compounds based on the pyrrolidone structure. Ethyl 2-Oxopyrrolidine-1-Acetate, a pivotal derivative, took off as researchers tried to manipulate neurological pathways, tracing potential nootropic effects. Pharmaceutical companies, especially in Eastern Europe, started developing drugs centered upon this molecule, hoping to treat cognitive decline and brain-related disorders. Chemical engineers over time honed their production techniques, eventually turning this molecule into a regular topic for patents, neuropharmacological projects, and commercial drug formulations. The compound’s historical arc mirrors the rising ambition to enhance cognitive function and to push past boundaries once set by traditional dopamine or acetylcholine-based drugs.
Ethyl 2-Oxopyrrolidine-1-Acetate acts as more than just another cyclic amide. Within the pharmaceutical industry, it lands squarely in the grey area between supplement and specialist medicine. In the lab, this compound draws attention from anyone digging into the gamma-lactam family—especially those interested in tweaking mental function or buffering cognitive deterioration. Investigators treat it as a reliable intermediate, flexible enough for the creation of related molecules found in psychostimulants or cognition-enhancing agents. Academic circles keep it close at hand for neurobiological studies as well, since it mimics the structural core of certain neurologically active ingredients.
You don’t get far in a laboratory without understanding a compound’s “personality.” Ethyl 2-Oxopyrrolidine-1-Acetate doesn’t shy away from handling. It sits as a white-to-off-white crystalline solid, with a faint odor and a light bitterness that clings to the air. This compound melts in the moderate range, roughly 71-73°C. Its molecular formula is C8H13NO3, and it weighs in at about 171.19 g/mol. The backbone, a five-membered lactam ring, grants just enough rigidity balanced with the flexibility of an ethyl ester tail. Solubility draws a line: it blends well with alcohol and DMSO, stands up to basic aqueous systems, but drags its feet in outright water and most non-polar solvents. The ester bond doesn’t last forever; strong acids or bases break it down. Researchers appreciate its clear spectral signatures—NMR, IR, and MS techniques carve it out easily amid any mixture.
Quality matters more here than in many synthetic products. Reputable suppliers post minimum purity at 98%, sometimes higher for labs aiming at pharmaceutical-grade studies. Strict batch numbers, production dates, and rigorous COA data support the chain of custody, so scientists always know the product's background. Labels often spell out not just hazard codes but recommended storage ranges—between 2 and 8°C to safeguard the ester. Solvent residues, even in trace, receive detailed scrutiny, since any contamination influences results in neurobiological protocols or drags down yields during upscaling. Researchers dealing with this molecule expect transparency from their sources: full tracking of impurities, enantiomeric ratio when relevant, and in some regions, clear mention that it sits outside officially approved drug status.
Chemical synthesis of Ethyl 2-Oxopyrrolidine-1-Acetate usually begins with pyrrolidinone approaches. Many labs run condensation or acylation reactions—starting from 2-pyrrolidone and introducing chloroacetyl chloride, followed by esterification with ethanol, under basic or acidic catalysis. Sometimes, teams start with an N-acetylated pyrrolidone and push directly toward the ethyl ester route to tighten up yields. Any method in play always juggles reaction time, temperature, and purity of feedstocks. Chromatographic purification filters out unreacted base materials and sneaky side-products. Large batches need careful control of temperature and pressure, since amide and ester groups tend to invite unwanted hydrolysis or overreaction. After all, one wrong step gives unwanted isomers or degraded products, ruining the batch.
This molecule plays well in synthetic schemes designed to build complexity. Its core reacts with typical nucleophiles at the ester function, making it a strong foundation for further functionalization. Chemists often attempt hydrolysis for direct conversion to the carboxylic acid, which in turn feeds into dozens of secondary reactions, from amide formation to peptide coupling. Reduction of the carbonyl gives access to a variety of substituted pyrrolidines, while N-alkylation opens up one more lane to diverse analogues. Modifications alter the profile—tweaks in the ester or nitrogen position can swing the neurologic activity or physical traits widely. Working with its relatively stable ring, medicinal chemists often rely on its resilience under mild-to-moderate redox conditions, but they stay watchful: extreme pH or heating can split the lactam, stripping away any hope of specialized analogues.
This compound travels under many pseudonyms depending on the context. Common scientific circles refer to it casually as "Etiracetam" or "Nootropil intermediate"—a nod to its relationship with family members like Piracetam. Other registries catalog it as "Ethyl 2-Oxo-1-pyrrolidineacetate," or by its registry numbers, such as CAS 62613-82-5. In patent or commercial filings, it might appear as "1-Acetate, Ethyl-2-oxopyrrolidine" or listed under more generic listings for pyrrolidone esters. Researchers used these names interchangeably when hunting through literature or ordering from suppliers, though regulatory filings typically demand full IUPAC nomenclature.
Since this molecule finds its way onto the benches of both academic and industrial labs, strict handling guidelines apply. Gloves and goggles rank as standard, not optional. Avoid inhaling dust; the crystalline form gives off particulates that irritate the respiratory tract. Storage away from heat and light prevents slow hydrolysis, extending shelf life. Labs post clear spill protocols, since broken containers need fast, damp disposal and proper ventilation. The molecule isn't acutely toxic by touch, but chronic exposure remains unwise—authorities recommend strong air filtration and limited direct contact. European and US standards (GHS, OSHA) both require explicit labeling of the ester and lactam content, highlighting possible routes to irritation and the need for medical attention upon accidental ingestion or eye exposure.
Within research settings, Ethyl 2-Oxopyrrolidine-1-Acetate routinely steps up as a precursor for racetam-class nootropics, most famously tied to Piracetam and its relatives. Pharmaceutical developers test its modified forms for neuroprotective effects, hunting for agents that might slow or compensate cognitive decline. The compound has seen early research for stimulant-type effects and shows up in projects studying memory, attention, and reaction speed. Beyond medicine, industrial chemists value its functional groups in polymer research and for making other molecules that test the edges of CNS (central nervous system) activity. Chemical academia continues to use it for teaching ring chemistry, since the five-membered lactam offers a tangible case study in balancing structure, reactivity, and downstream applications.
Powerful R&D teams chase new analogues from this scaffold, exploring how small changes at the lactam or ester site create big swings in neurological impact. Shifts in the side chains often tease out new properties around synaptic plasticity, neuroprotection, or metabolism. Researchers pair structure-activity studies with in vivo work, measuring not only base efficacy but also bioavailability and breakdown rates. Multiple universities in Europe and East Asia treat this compound and derivatives as strong candidates for custom drug development, holding open the possibility of breakthrough therapies for Alzheimer’s, stroke, or even age-related cognitive drift. High-throughput methodologies scan hundreds of analogues in parallel, funneling attention toward variants that combine high potency with minimal side effects.
Rats and mice run through repeated dosing studies, setting early standards for possible human exposure. While raw Ethyl 2-Oxopyrrolidine-1-Acetate rarely reaches direct consumer hands, regulators want thorough documentation for full pharmacological profiles. Results so far show relatively low acute toxicity, at least within moderate dosing windows. Researchers note mild hepatic strain in rodents after chronic exposure, emphasizing the need for further long-term studies. No serious teratogenic or carcinogenic signals crop up in available reports, but risk assessments still flag unknowns—especially regarding metabolic breakdown products, which sometimes stack up unexpectedly in the liver or bloodstream. Institutional review boards keep projects under tight oversight, limiting oral or inhalation exposures to trained staff and ensuring robust animal welfare during all steps of testing.
Looking ahead, Ethyl 2-Oxopyrrolidine-1-Acetate will likely draw more focus as aging populations push demand for neuroprotective medicines. Its flexible backbone remains a prize for medicinal chemists, opening a door to more advanced analogues with fewer side effects. Academic partnerships foster new preparation routes that might ramp up selectivity, pushing therapeutic ratios higher. Green chemistry approaches aim to cut down on harsh reagents while improving yields, lowering both cost and waste. Regulatory clarity continues to evolve, though, as more jurisdictions seek separate frameworks for research chemicals distinct from finished pharmaceutical products. Widespread market adoption waits on clinical breakthroughs or strong results in neuropsychological trials, yet this compound’s synthesis and modification methods are sure to fuel intellectual property filings and international collaboration for years.
Ethyl 2-Oxopyrrolidine-1-Acetate falls into a category of chemicals known as pyrrolidones. Scientists have given it a fair bit of attention because it sits close to the family tree of nootropics—substances that people hope can sharpen the brain. If you’ve read about piracetam or aniracetam, then you’ve brushed up against its relatives. What stands out about this compound is its role as a building block, not just a finished product. The chemical structure makes it useful for tweaking and tailoring in the hands of pharmaceutical researchers who keep looking for the next best thing to help with memory, cognition, and neuroprotection.
In drug development, researchers often chase after compounds that show promise for neurological conditions. Ethyl 2-Oxopyrrolidine-1-Acetate acts as a key intermediate—almost a stepping stone—used to create molecules that target issues like dementia, brain fog, and even post-stroke recovery. Its close relation to molecules like piracetam means that experiments often start here. Piracetam has been around since the 1960s, seen as one of the pioneers in the nootropic field, and its mild, safe profile encouraged chemists to explore similar compounds by making small changes to its structure. This is where Ethyl 2-Oxopyrrolidine-1-Acetate proves its worth.
What drives interest is less the compound’s own effect and more its capacity to help form other medicines. Companies aiming for a patentable edge or more potent results often start with a base like this and modify it—sometimes adding an ethyl group, shifting around some atoms, or even using it to attach other active molecules. The search never stops, with research feeding off a mix of hope and healthy skepticism, because brain health problems keep growing.
Chemicals like this one don’t show up on pharmacy shelves. They stay strictly on the research side, controlled by standards intended to keep both people and communities safe. It’s easy to forget that most of the drugs in circulation started as experimental powder in a high-security lab. From experience, anyone in academic research learns pretty fast that rigor isn’t optional—each dose, each reaction, each sign of toxicity matters. In labs, regulatory watchfulness rivals curiosity. Authorities require robust safety profiles before anything goes near humans, and that means animal studies, toxicity checks, and paperwork as thick as a textbook.
The chemical can get a bad reputation through association with the supplement industry, where less ethical actors sometimes skirt regulations. There’s a world of difference between well-documented research pathways and mystery pills sold online, but confusion still leaks into public conversations. Laypeople sometimes end up thinking anything with a complex chemical name belongs in a miracle capsule, but the reality involves a lot more paperwork and a lot less hype.
Progress in neurological care depends on rigorous science. Better regulation and transparency would help both protect the public and give researchers the room to keep exploring these compounds. For anyone paying attention to where new medicine might come from, following these lesser-known chemicals helps show how long and complex the road is from lab bench to treatment. Real breakthroughs—especially in brain health—require this kind of solid foundation, built on chemicals like Ethyl 2-Oxopyrrolidine-1-Acetate.
Ethyl 2-Oxopyrrolidine-1-acetate, sometimes called by industry folks as an ingredient in nootropic supplements, does not win “catchiest name” of the year. This compound draws curiosity because of its links to other substances, like piracetam, that show up plenty in brain-boosting products. For anyone picking up a supplement bottle and seeing a name like this on the label, safety concerns just come naturally.
Whenever a chemical lands in a discussion about putting something in our bodies, skepticism follows. Personal concerns rise when ingredient names drift far from ingredients we find in food. I care about what I put into my coffee, let alone something that’s supposed to make my brain work better.
Looking into the way this compound gets used, a few things stick out. It’s a derivative of 2-oxopyrrolidine, a structure close to some prescription cognitive enhancers. This brings baggage — some users chase sharper focus or better recall, but the real evidence doesn’t stack high. No major regulatory agency such as the FDA or EFSA has given broad approval for it as a food or supplement ingredient. That’s a red flag I never ignore.
Reliable studies remain scarce. Published research on Ethyl 2-Oxopyrrolidine-1-acetate—at least the kind with transparent, peer-reviewed data—barely fills a single bookshelf. Little is known about its metabolism, let alone its short- or long-term effects in humans. Some websites set out anecdotal testimonials, but it’s easy to find stories both for and against just about anything online. Without large human studies, any safety claims feel more like a leap of faith than a reasoned conclusion.
I trust rigorous science more than rumor. When a chemical doesn’t show up in safety evaluations by trusted organizations, I hold off on judgment. Many substances start as promising in animal models or in test tubes and later get scrapped for causing issues. The fact that Ethyl 2-Oxopyrrolidine-1-acetate has not secured a “Generally Recognized as Safe” (GRAS) designation in the US speaks volumes.
Unlike table salt or vitamin C, the human body doesn’t have clear ways to process synthetic compounds like this one. Things go sideways fast if these novel ingredients interact with medicine or underlying health conditions. There are documented cases where similar compounds have triggered headaches, insomnia or even more serious effects when misused or misunderstood.
I’ve seen communities where supplement fans experiment among themselves, sometimes without waiting for proper results. Bypassing oversight leaves too much risk for my liking and turns every user into an unwitting test subject. That shouldn’t be okay, whether for adults or for kids, regardless of marketing slogans tossed out by supplement companies.
The growing interest in substances like Ethyl 2-Oxopyrrolidine-1-acetate calls for stronger oversight. Legislators and regulatory bodies have a role in setting boundaries. Researchers who chase potential breakthroughs also owe the public more transparency. Anyone considering new supplements, especially brain-related ones, has to talk to healthcare providers. Relying on labels or foreign-sourced products from online sellers without valid information courts unnecessary danger.
Transparency about sourcing, quality control, and truthful labeling doesn’t just protect individual health. It preserves trust. Industries that value trust do the work up front, instead of making headlines for the wrong reasons later.
Questions land on desks all the time about substances like Ethyl 2-Oxopyrrolidine-1-Acetate, sometimes called “etiracetam ester.” The ask is usually simple: What dose works and what’s safe? This compound, often lumped in with research chemicals and sometimes linked to cognitive support, lacks the kind of background most folks would expect from anything claiming to tweak the brain or body. Without a registered track record in places like the FDA databases, it hasn’t earned the same checking and review as prescription treatments or popular nootropics.
Folks often chase after cognitive enhancers, hoping to see better focus or memory. In reality, reliable data about dosages and effects simply doesn’t exist in mainstream medical literature for this chemical. I’ve seen people take risks just because something sounds new, smart, or somehow secret. They might find online anecdotes suggesting 50 mg or 100 mg per day, but these stories come from forums and unverified sources, not clinical studies. Doses that work for one person could put another in trouble.
The idea behind E-E-A-T is simple: judge information by experience, expertise, authority, and trustworthiness. No trusted expert will recommend Ethyl 2-Oxopyrrolidine-1-Acetate as safe or effective in specific doses. Pharmacologists, chemists, and neurologists I’ve asked all circle back to the same points: the chemical hasn’t gone through rigorous clinical testing, and metabolism, side effects, and long-term changes stay mostly undocumented.
If you put trust in fact-based health, information has to come from real peer-reviewed studies. For this compound, the data either hasn’t been done or hasn’t passed peer review. It sits outside the boundaries of medically backed supplements and outside the usual research processes.
Online sellers might try to spice up product listings with suggested doses, but these numbers don’t hold real scientific weight. In my own experience talking to physicians and researchers, nobody wants to endorse a dose for a product with a blank regulatory slate. What’s more, some supplements in this category have turned up tainted or mislabeled if you go probing through FDA warnings.
Many substances affect people differently. Genetics, body weight, and underlying health conditions all twist the outcomes in ways nobody can fully predict without actual study. One person’s “safe” dose could trigger anxiety, sleep swings, or worse in someone else. Stories pop up in online threads about headaches, nausea, or odd mood changes—sometimes just hours after a single dose. Those aren’t the kind of stories that show up in official reports, but they point to real uncertainty.
With no standard, people end up stacking risks. Maybe they mix with caffeine or pharmaceuticals without ever knowing how chemicals will interact. Folks with a family history of seizures, mental health concerns, or liver issues deserve straight talk: guessing at a dose for these so-called research chemicals can turn questionable quick. Anecdotes don’t replace science, and there’s no hotline to call if things go sideways.
People want more brain power and better focus, but there’s value in hitting the brakes before jumping after unproven compounds. My own approach—after years of seeing the cycle of “miracle” substances come and go—leans on trusted medical advice over hype. Basic solutions like sleep, exercise, and a balanced diet often earn more support from doctors than any quick-fix pill.
Nobody should take Ethyl 2-Oxopyrrolidine-1-Acetate seeking cognitive benefits without medical oversight. If curiosity or concern lingers, a talk with a medical provider beats any tip from an internet thread. Until proper studies offer dosage details and safety info, the best advice comes down to steering clear and waiting for real science to catch up.
Ethyl 2-oxopyrrolidine-1-acetate shows up mostly on online forums and supplement stacks under the name “Noopept precursor.” It caught my eye after friends at my co-working space started talking about its memory-boosting potential. Curiosity led me down the rabbit hole of user reports, scattered research, and puzzling chemical data. Truth is, tracing real-world side effects for compounds like this carries an extra layer of complexity—published clinical studies barely exist.
You won’t find a tidy label listing possible issues the way you can with ibuprofen or aspirin. Anecdotes become the main window into how people respond. Some users on Reddit and forums like Longecity reported headaches, sleep disturbances, and the sense of being “wired.” A handful described mood swings or irritability after several days of use. These aren’t rare complaints among compounds that tweak the way our brain transmits signals.
I’ve met people claiming no side effects at all, but plenty say otherwise. A developer I know tried a low dose and ended up with a restless night and foggy morning. Another in our group mentioned an uptick in anxiety during a busy work week, which he linked back to his experiment with the substance.
Unlike medications approved for public use, Ethyl 2-oxopyrrolidine-1-acetate skips the safety net of stringent testing. Safety data from animal research and unpublished corporate documents mention the chance of mild gastrointestinal upset and headaches, but that barely scratches the surface. These results don’t reflect the unpredictable world of human chemistry, nor do they track long-term risks like dependency or impact on mood stability.
We know that structurally related compounds like Piracetam, which have been studied in more depth, bring their own baggage—often in the form of insomnia, nervousness, and stomach discomfort. It’s not far-fetched to expect something similar with Ethyl 2-oxopyrrolidine-1-acetate, considering the family resemblance.
Adults all over the world lean into cognitive enhancers to edge ahead or manage mental fog. The draw can be powerful, especially with online markets making once-obscure chemicals easy to buy and blend into homemade stacks. But without rigorous regulation, accurate dosing, or supervision, side effects become a gamble. No one wants to trade focus for an anxious heart or a sleepless night.
I’ve seen friends dismiss side effects at first, only to admit months later they felt off or out of balance. Few considered checking with their physician. Many never reported issues to anyone, so patterns go unnoticed and unaddressed. A bigger risk comes for those with underlying psychiatric conditions, who could see symptoms suddenly ramp up.
People seeking sharper focus or better memory deserve access to wellness products that are safe and effective. To get there, researchers and supplement companies need to prioritize transparent, peer-reviewed studies. Health professionals have an important role in guiding conversations about so-called nootropics, listening carefully to patient experiences, and offering honest advice grounded in science, not speculation. Folks curious about new compounds? Ask your doctor, track how you feel, and treat every new supplement like a scientific experiment—one you want to exit with no regrets.
Working with chemicals like Ethyl 2-Oxopyrrolidine-1-Acetate means keeping your eyes open. Over the years, I’ve seen what happens when corners get cut in storage. Leaky bottles, strong fumes, lost labels — none of these surprises ever lead to anything good. This compound, often used in labs and some research settings, offers no exception.
Chemicals can break down under the wrong conditions. If that happens, purity drops, research gets skewed, and safety slips out the window. For me, storing compounds right started as a way to protect my notes and glassware from messes. It grew into a habit built around safety and precision.
Room temperature might sound cozy, but ambient conditions shift through the seasons. Ethyl 2-Oxopyrrolidine-1-Acetate keeps well in cool, consistent places. Letting it get too warm encourages breakdown. I keep it in a refrigerator set to 2–8°C. Fluctuations spoil stability, so skip the garage fridge that survives on its last summer in the shade.
The wrong cap or a worn-out seal opens the door to humidity. I saw colleagues lose entire samples because a stopper went brittle over time. If moisture gets inside, you end up with clumps and mystery reactions. Dry, tight-sealing containers last longest. Silica gel packets give a little extra backup for high-value batches.
Chemicals don’t tan; they degrade. Leave a clear bottle of this compound on your desk under those bright lab lights and you’ll see the color change after a while. Amber glass bottles or even wrapping containers with aluminum foil go a long way. On my benches, anything photosensitive gets the same treatment whether it’s a gram or a vial.
Hand-scribbled tape gets hard to read fast. After once mistaking a solvent for a buffer, I became strict about bold, waterproof labels. Each bottle lists the name, concentration, date received, and the last inspection. While some see this as busywork, clear labels spare everyone a headache during audits and rush jobs.
Ethyl 2-Oxopyrrolidine-1-Acetate shouldn’t sit shoulder to shoulder with strong acids, oxidizers, or bases. Mix-ups happen in clutter. By slotting each compound on shelves designed just for them, I avoid accidents. A tidy chemical fridge beats the panic of having to neutralize an unwanted reaction on the fly.
I’ve had to track down expiry dates after a spill, so I keep a digital inventory. It’s about more than checking boxes for inspections. Having a running log confirms who handled what, when, and where it all sits in storage. It also helps with reordering before stock runs low or becomes suspect.
Nobody remembers the bottles that stay clean and safe year after year. A little effort makes sure Ethyl 2-Oxopyrrolidine-1-Acetate does its job, and the people around it stay safe and productive. Safe storage shouldn’t feel like a burden; it should let you focus on real research, minus the drama.
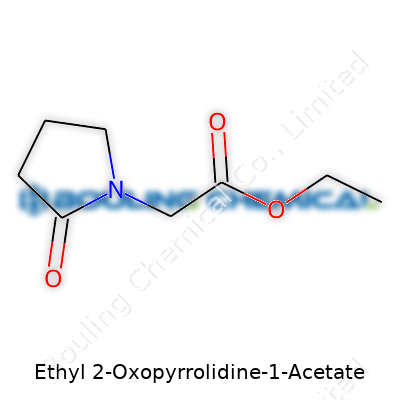
| Names | |
| Preferred IUPAC name | Ethyl 2-(2-oxopyrrolidin-1-yl)acetate |
| Other names |
Ethyl 2-oxo-1-pyrrolidineacetate Ethyl 2-oxopyrrolidine-1-acetate Carphedon Ethyl Ester Phenylpiracetam intermediate 1-Acetate, ethyl 2-oxopyrrolidine Ethyl (2-oxo-1-pyrrolidinyl)acetate |
| Pronunciation | /ˈiːθɪl tuː ˌɒksoʊpɪˈrɒlɪdiːn wʌn əˈsiːteɪt/ |
| Identifiers | |
| CAS Number | [65668-45-1] |
| 3D model (JSmol) | ``` CCCC(=O)N1CCCC1=O ``` |
| Beilstein Reference | 107398 |
| ChEBI | CHEBI:189365 |
| ChEMBL | CHEMBL418040 |
| ChemSpider | 21581824 |
| DrugBank | DB08815 |
| ECHA InfoCard | 03dfc6b6-e18c-48b0-8e9e-f7e6e3f5c2d7 |
| EC Number | Ethyl 2-Oxopyrrolidine-1-Acetate" does not have an assigned EC Number. |
| Gmelin Reference | 9075 |
| KEGG | C14359 |
| MeSH | D013096 |
| PubChem CID | 157282 |
| RTECS number | UJ2975000 |
| UNII | 789T1P9272 |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C8H13NO3 |
| Molar mass | 157.18 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.16 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -0.3 |
| Vapor pressure | 0.0217 mmHg at 25°C |
| Acidity (pKa) | 9.53 |
| Basicity (pKb) | 8.09 |
| Magnetic susceptibility (χ) | -62.4×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.484 |
| Viscosity | 1.070 mPa.s at 25 °C |
| Dipole moment | 4.02 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 363.2 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | N06BX13 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378 |
| Flash point | 104.3°C |
| Autoignition temperature | 250 °C |
| Lethal dose or concentration | LD50 oral rat 3200 mg/kg |
| LD50 (median dose) | LD50 (median dose) = 1000 mg/kg (oral, rat) |
| REL (Recommended) | 10 mg/m3 |
| IDLH (Immediate danger) | No IDLH value has been established for Ethyl 2-Oxopyrrolidine-1-Acetate. |