The path to Methyl 2-Aminothiazole-4-Carboxylate traces back to the boom days of organic chemistry in the mid-20th century. Back then, researchers at universities and chemical companies chased heterocyclic compounds for new medicines and crop-protection agents. The thiazole ring, core to this molecule, showed up in early antibacterial drugs and enzyme inhibitors. Over decades, labs honed its synthesis and found fresh uses, moving from curiosity status to a critical building block for pharma and material science. Factory production kicked off in earnest in the 1980s, allowing for research at a scale nobody pictured at the time.
You often find Methyl 2-Aminothiazole-4-Carboxylate tucked away in the catalogues of specialty chemical suppliers, usually as a white or off-white powder. Its value comes from the tricky thiazole ring, which pulls in synthetic chemists. Add a methyl ester and an amino group, and suddenly the molecule can slide into all sorts of reactions. While its name sounds complex, researchers see it as a versatile node—a compound that ties together a lot of reaction pathways.
On the lab bench, this compound weighs in with a molecular weight of around 172.2 g/mol. It dissolves better in alcohols and organic solvents than water, thanks to the aromatic thiazole core and the methyl ester. Its melting point hovers close to 90°C, sometimes lower if impurities sneak in. The amino group brings its own punch, making the molecule more reactive—handy for chemists looking to tack on all kinds of chemical groups. The compound has a slight, sharp odor, not uncommon in low-molecular-weight organics, enough to remind you to keep the lab ventilated.
Bottles usually come labeled with purity grades, CAS registry number (13241-41-9), and storage advice. For solid samples, you’ll see “store below 25°C, dry environment.” Analytical data—NMR, IR, HPLC—help confirm the identity, since thiazole derivatives can trip up even trained eyes with similar-looking powders. Typical product sheets spell out whether the sample includes any residual solvents or water by Karl Fischer titration. Some suppliers provide certificates of analysis with every batch, which adds trust at the bench.
labs often cook up Methyl 2-Aminothiazole-4-Carboxylate from thioamide derivatives and methyl esters, usually by cyclocondensation reactions. One handy way involves treating ethyl cyanoacetate with thiourea and methyl chloroformate, then careful work-up and purification. Other routes swap methyl chloroformate for dimethyl sulfate or use safer, greener solvent systems to produce the compound at scale. Yields swing from 60% to 90%, depending on the operator’s skill or the subtleties of the setup. Purity depends on clean handling—skip a step, and things get ugly with by-products and unreacted starting material.
The amino group invites all sorts of acylation, alkylation, and even Suzuki coupling if you modify the scaffold right. Medicinal chemists latch onto that group to build libraries of potential drugs, since attaching different aryl or alkyl groups at that site can change how a compound interacts in the body. The methyl ester opens the door for hydrolysis—run it in base, and you land the free acid. More adventurous hands might transform the ring itself, building thiazole-fused bicycle systems or pushing oxidative couplings for richer skeletons.
In chemical catalogues, you can find Methyl 2-Aminothiazole-4-Carboxylate under a few aliases: Methyl 2-amino-4-thiazolecarboxylate, Methyl 4-carboxylate-2-aminothiazole, or sometimes as its CAS number 13241-41-9. Different suppliers brand it with their trade names or stock codes, but as always, a smart researcher double-checks the structure before ordering.
Chemists learn early that thiazole esters need ventilation and gloves. Although its acute toxicity profile seems manageable, the powder can irritate skin and eyes, and inhalation brings coughing or a sore throat. Labs follow standard precautions: nitrile gloves, fume hoods, eye protection. Disposal routes treat residues as hazardous waste, never down the drain. Spill kits stand by for powders, since the thiazole ring can be caustic or even allergenic with repeated exposure. Material Safety Data Sheets recommend routine washing of hands after use, and strict record-keeping prevents accidental mixing with incompatible chemicals.
Medicinal chemists turn to this compound for drug design—antibacterial, antifungal, and central nervous system-related compounds all trace their routes through thiazole systems. The methyl ester group provides an anchor for further modification, making it attractive in combinatorial chemistry where hundreds of analogs must be churned out quickly. Beyond drugs, some researchers push the molecule into dye chemistry and materials science, where the heterocycle alters electronic properties or binds metals in catalysts. Agricultural science and crop protection research look for novel agents using this as a starting point, as they chase new ways to disrupt harmful pests.
Research on Methyl 2-Aminothiazole-4-Carboxylate keeps growing as new methods roll out for faster synthesis or greener chemistry. Pharma R&D teams push for higher throughput, developing microwave-assisted reactions or using solid-phase supports to speed up analog production. Structural biologists design inhibitors for everything from bacteria to cancer, tweaking the core thiazole for better selectivity and lower side effects. University labs and companies work hand-in-glove, with patents popping up for new analogs almost yearly. Open-source chemistry projects occasionally feature this compound as a scaffold for urgent disease treatments.
Toxicologists haven’t found overwhelming risks yet from this compound, compared to some nastier heterocycles; organ toxicity results from animal studies remain mild at moderate doses. Eye and skin contact do cause irritation, sometimes enough to halt an experiment for cleanup and medical checks. There’s no wide data showing potent carcinogenic or mutagenic effects, but lasting safety means fresh rounds of screening every time a new derivative gets made. Binding studies show thiazole rings might slip into biochemistry in unplanned ways, so labs remain wary, especially as molecules move toward trials or large-scale application.
Work on Methyl 2-Aminothiazole-4-Carboxylate will only ramp up from here. Green chemistry pushes for less hazardous solvents and milder reaction conditions, since chemical manufacturing faces more environmental scrutiny every month. Emerging diseases and resistant pathogens drive researchers to search for new treatments, prompting deeper exploration into thiazole compounds as unique enzyme inhibitors or antiviral agents. Material science, always hungry for strange new scaffolds, experiments with the electronic tweaks possible from this backbone. As someone who’s spent late nights troubleshooting weird NMR signals and tracking down impurity sources, I see the next round of innovations coming from a mix of traditional insight and new tools—automation, AI-driven chemistry, and sustainable reaction planning. Practical experience, attention to detail, and openness to unconventional ideas will carry this chemistry to tomorrow’s breakthroughs, just as the past shaped today’s methods.
Every chemist knows purity isn’t just a number penciled onto a certificate—it shapes everything from lab results to how smoothly a manufacturing run goes. Take methyl 2-aminothiazole-4-carboxylate, for example. Purity often runs in the ballpark of 97% or higher when you check suppliers’ technical sheets. But ask anyone who's spent late nights in a process lab, and they’ll tell you even those last few percent can make headaches or save the day, depending on what rides along as “impurities.”
Purity does more than impress quality managers. For this compound—and others used in pharmaceutical or fine chemical routes—unwanted leftovers show up in yields, downstream reactions, and even analytical headaches. Impurities may sneak through HPLC runs, stain NMR spectra, or throw LC-MS readings off. In my own experience, chasing ghosts from a barely-visible contaminant added weeks to an otherwise simple API route. We traced it all the way back to a single dodgy shipment of starting material.
Different lots of methyl 2-aminothiazole-4-carboxylate can arrive with the same headline purity, but minor side-products such as residual solvents, starting materials, or structural isomers still create issues. In some projects, tiny levels of sulfur- or nitrogen-based by-products produced strange odors or reactivity shifts. Purity, in that sense, isn’t always the whole story—knowing the impurity profile makes a real difference during scale-up or regulatory filings too.
Vendors often throw out claims: “99% pure by HPLC.” The truth: methods vary. Some lots look clean on one detection method and show a different picture on another. Back in a kilo-lab, we’d freeze work because thin-layer chromatography spots didn’t match vendor chromatograms. Each analytical tool sees different things, and documentation rarely lays out the full impurity picture. So, purity numbers without context lose value if you’re headed for tough reactions or final-dose work.
Every impurity avoided cuts filtration time, reduces waste, and smooths regulatory inspections. If I’ve stood sweating in a hot plant floor, trying to troubleshoot filtration gunk, it’s almost always tracked back to an “acceptable” impurity. The cost of super-pure methyl 2-aminothiazole-4-carboxylate pays for itself: fewer headaches, smaller safety margins required, less time reworking failed crystallizations.
Getting close to true chemical purity means grilling your supplier—ask for detailed analytic data, not just a number from generic testing. Request a full impurity profile, including solvents, isomers, and trace metals. The best vendors support batch-specific reports and respond when you question the numbers. In routine work, spot-check every batch with your own analytic methods. Keep a sample from “gold standard” lots and side-by-side test everything new. Time spent vetting sources pays off in fewer surprises.
No one expects every raw material to arrive at 100% purity, but ignoring what’s hidden in those missing few percent opens the door to surprises—some of them expensive or dangerous. Skill, experience, and a bit of stubbornness in chemical sourcing solve more problems than one more round of lab troubleshooting ever can.
Storage recommendations for any compound often get buried in the fine print, but ignoring those details brings risks. A lot of folks in science, research, or industrial settings have seen what goes wrong when people skip these steps. Chemical stability relies on more than just tossing a jar on a random shelf and slapping on a “don’t touch” label. The health of people, data, and property all tie back to simple storage decisions.
I ran into this issue working in the teaching labs during college. We often kept sensitive powders in a regular classroom cabinet, which seemed fine in the cool seasons. As summer hit, humidity and temperature crept up. That "fine white powder" started clumping, color shifted, and one day the safety officer flagged an off smell right where we stored these bottles. Big lesson—just a small temperature change can break chemical bonds or boost reactivity. Recommendations exist for a reason. Some need to live in a fridge, others in a freezer set to minus twenty, and a few prefer ordinary room temp, but away from any windows or vents.
Moisture loves to wreak havoc. Desiccators, those dry-box contraptions with silica gel, save lives for certain salts and pharmaceuticals—one missed day and you find puddles forming, crystals oozing, or samples dissolving on their own. Light can fade compounds, especially biological stains and some dyes. Keeping them in amber bottles or stashing them deep in cabinets, as funny as it sounds, actually blocks wavelengths that drive photochemical breakdowns. Most safety datasheets point this out, but habit matters more than memorization.
Labels aren’t cool or flashy, but they steer people away from catastrophic mix-ups. Clear, permanent writing, with date received, open date, and hazard symbols, makes mixing errors rare. Once, a chemistry club I joined tried to save money by reusing old bottles. We nearly mixed nitric acid with ethanol, which would have ended badly. Proper labeling and separate storage for acids, bases, and organics minimize accidents—the details tell a story that makes sense to anyone walking by, not just the expert.
Plenty of us deal with cramped storage rooms, packed fridges, and little extra space. Even there, good habits help. Shelves set for chemical compatibility, frequent inventory checks, and ditching expired or unused materials are straightforward ways to keep things safe and stable. Using sturdy containers and secondary containment—just plastic bins with tight lids—stops small spills from turning into full-on cleanups. It’s not rocket science. Most disasters happen because those basics slipped off someone’s radar.
Storage guidelines aren’t just box-checking exercises from bureaucrats. Decades of accidents and experiments built these rules. By following temperature, humidity, light, and segregation rules, you keep compounds usable and avoid harm. Whether it’s research-grade solvents, biological samples, or specialty catalysts, biting the bullet and storing them by the book saves time and trouble down the road.
People often pass over chemistry with a groan, picturing long equations and endless calculations. Yet, every material, every breath we take, boils down to the tiniest building blocks—molecules. Knowing what makes up a molecule and calculating its weight brings answers to all sorts of questions, from solving health problems to stopping pollution.
The formula for a molecule breaks down its makeup, showing how many atoms of each element join together. Looking at water, the formula H2O clearly means two hydrogen atoms connect with one oxygen. That tiny bit of difference in a formula can turn sugar into alcohol, or safe oxygen into poisonous ozone. Chemists see these formulas as blueprints, guiding their hands as they build new drugs, plastics, batteries, or fertilizers. No formula, no clear path; scientists end up guessing rather than creating.
Grab a box of fever medicine or a bottle of bleach. Somewhere, the molecular weight hides out in the fine print. This number tells pharmacists how much of an ingredient to mix so patients get the right dose—not too much, not too little. Paint manufacturers measure molecular weights to make sure their products spread just the way painters want. Soil scientists use these numbers to predict if a pesticide will stick around in the dirt or get washed into water supplies. People tend to ignore these calculations, but they shape safety labels, delivery costs, and even business profits.
Back in college, I once ran a chemistry experiment needing pure sodium chloride—table salt. The protocol asked for five grams, but rushing through things, I mistook the formula and nearly grabbed sodium carbonate, a common cleaning agent. I caught myself in time, but mixing the wrong chemicals that look similar can ruin an experiment or, worse, cause a dangerous reaction. The right formula isn’t just a string of letters; it draws a line between success and failure, health and harm.
Despite digital calculators and endless internet resources, mistakes pop up all over. Students flip numbers or mix up symbols. Seasoned professionals, pressed for deadlines, might trust a supplier’s label without double-checking. Even advanced research teams sometimes overlook a single atom here or there, throwing off findings and wasting months of work. It rings true outside the lab, too. The infamous melamine milk scandal in China came down to faked molecular data that masked contamination. Misinformation cost children their lives and laid waste to a massive industry’s reputation.
Clear chemical formulas and accurate weights help avoid costly errors. Schools should bring real-world cases into the classroom, turning theory into hands-on practice—let kids see chemistry in action, not just on tests. Companies could use double-check systems before shipping products, much like pilots checklists before takeoff. Everyday users—gardeners, cooks, mechanics—could benefit from easy-to-read charts instead of cryptic codes on packaging. Better labels and quick reference guides, right at the shelf, might save nerves, money, and even lives.
At the end of the day, every household cleaner, every pill, every snack in the grocery bag traces back to these basic numbers. Learning them well, using them right, steers us clear of dangerous mix-ups and keeps life humming along safely.Every year, more folks ask, “Is there a COA available for this product?” It shows how trust in companies isn’t automatic anymore. Shoppers want clearer proof about what they’re buying, especially with everything from supplements and CBD oils to protein powders flooding the market. Years ago, lots of products arrived in plain packaging, and nobody thought twice. Things changed once stories started popping up about fake ingredients and contaminants sneaking in. Skepticism comes from experience, not nowhere.
A Certificate of Analysis, or COA, is basically a third-party receipt that confirms what’s listed on the label matches what’s in the container. If you ever bought vitamins that didn’t smell right, or protein powder that didn’t mix the same as the last bag, you learned that labels don’t always tell the whole story. I remember picking up a bottle of melatonin once that did nothing for my sleep—turns out the batch had almost no active ingredient in it. Seeing the actual test results gives customers a way to hold brands accountable, which feels long overdue.
I used to scoff at the idea of checking paperwork for health products, trusting big logos on shelves. That mindset almost got me into trouble. A close friend landed in the ER because an “all-natural” supplement contained undisclosed pharmaceuticals. If her bottle came with a public COA, that hidden ingredient likely would have been caught long before. Now, I always look for third-party lab reports—especially with anything you ingest or put on your skin.
Some brands dodge the COA question or hide behind vague promises. That’s a big red flag. If a company is unwilling, or “waiting” to share test results, it often hints at cost cutting or corners being shaved. Plenty of good companies publish their COAs right on their website, batch by batch, with clear explanations. It’s not magic—every trustworthy manufacturer gets laboratory screening by default, and digital copies are easy to post online. If the information isn’t there, it begs the question: what are they hiding, and why?
In the past, sharing lab tests was rare. Now it can make or break a business, especially in health, nutrition, and eco-products. People talk, share screenshots, and call out brands lacking transparency. For those of us with allergies or health conditions, seeing those test reports isn’t just satisfying—a simple result sheet turns a gamble into a confident choice. For parents, or someone supporting an elder on supplements, that extra step is worth gold.
The process has improved, but COAs need to be easier to find and read. Too many reports look like a jumble of chemistry jargon. Imagine if you could scan a QR code and see a plain language summary: is it safe, what exactly is in it, did it pass for things like heavy metals or bacteria? Companies who invest in this kind of open, clear communication build real loyalty. Regulators could nudge things forward by asking for public, standardized reports. Until then, keep asking for the COA. It’s a simple question that has the power to shift the whole industry, one batch at a time.
Methyl 2-Aminothiazole-4-Carboxylate might sound like something only chemists care about. In reality, it quietly supports several fields that touch daily life, from treating illness to pushing forward new tech in agriculture and industry. It’s not a household name, but the roles it plays often turn out critical behind the scenes.
Drug discovery remains a high-stakes game, and this compound pops up over and over on lab benches. Chemists use it as a building block for synthesizing more complex molecules. Ask anyone who’s spent time in pharmaceutical R&D—they remember late nights testing combinations to beat bacteria or control inflammation. Methyl 2-Aminothiazole-4-Carboxylate helps form thiazole scaffolds, which show up in a range of antibiotics and anti-inflammatory drugs. Those thiazole rings make molecules sturdier and more effective at targeting bacteria or easing chronic conditions.
I’ve watched colleagues tinker with thiazole carboxylates to chase new ways to block pain without nasty side effects. One day it’s about treating rheumatoid arthritis, the next it’s searching for better cancer therapies. A single modification to a molecule like this can mean the difference between a new breakthrough and a failed test. Researchers like flexibility—the kind methyl 2-aminothiazole-4-carboxylate offers.
Most people don’t think about where their food comes from beyond the grocery shelf, but chemistry shapes how crops resist pests and disease. Agricultural scientists often start with methyl 2-aminothiazole-4-carboxylate when working on next-generation pesticides or fungicides. Pests evolve, and so do weeds and plant-borne pathogens. Chemists combine these thiazole derivatives with other molecular fragments, testing what can knock down bugs or fungus but leave crops untouched.
I’ve seen research projects use this thiazole derivative to craft compounds that protect wheat, rice, or soy from blight and rot. The best part—many new thiazole-containing agrochemicals break down more easily in soil, which means less build-up and lower risk to water and wildlife compared to some older products.
Outside medicine and agriculture, this compound finds its way into dye and pigment manufacturing. Specialty dyes don’t just color clothes—they support everything from research to electronics production. The thiazole core serves as a starting point for colorants that hold up under heat and light.
In my early career, I worked with a team developing anti-corrosive coatings. We explored how thiazole carboxylates like this protect metal surfaces. Oils and gases might not seem glamorous, but pipelines need robust guards against rust and corrosion. The thiazole ring can bind tightly to metal, forming a barrier that holds up over time.
Across these industries, one challenge stands tall: reducing environmental impact. Chemists keep digging for ways to make synthesis cleaner and safer. Using renewable raw materials, recycling solvents, and lowering waste have real gains for both health and the planet. Companies can push for responsible sourcing and better waste management.
Smaller-scale production in research and specialty chemical companies keeps the door open for innovation. Open access to chemical databases and international collaboration have brought faster progress in medicine and crop science, and methyl 2-aminothiazole-4-carboxylate continues to open new doors as a result. The future promises smarter use of these fundamental molecules, with safer, greener chemistry guiding every step.
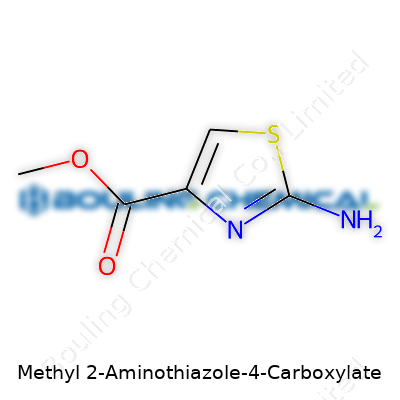
| Names | |
| Preferred IUPAC name | Methyl 2-aminothiazole-4-carboxylate |
| Other names |
Methyl 2-amino-1,3-thiazole-4-carboxylate 2-Amino-4-thiazolecarboxylic acid methyl ester |
| Pronunciation | /ˈmɛθɪl tuː əˈmiːnəʊˌθaɪəˌzoʊl fɔːr kɑːrˈbɒk.sɪ.leɪt/ |
| Identifiers | |
| CAS Number | 29445-60-1 |
| 3D model (JSmol) | `3Dmol.js?cid=71065611` |
| Beilstein Reference | 147544 |
| ChEBI | CHEBI:91299 |
| ChEMBL | CHEMBL185602 |
| ChemSpider | 177410 |
| DrugBank | DB08226 |
| ECHA InfoCard | 100.152.058 |
| Gmelin Reference | 736267 |
| KEGG | C05985 |
| MeSH | D000000 |
| PubChem CID | 120777 |
| RTECS number | XU2960000 |
| UNII | X6BXD7NZ8D |
| UN number | Not regulated |
| CompTox Dashboard (EPA) | DTXSID3040860 |
| Properties | |
| Chemical formula | C5H6N2O2S |
| Molar mass | Molar mass: 172.19 g/mol |
| Appearance | Light yellow solid |
| Odor | Odorless |
| Density | 1.4 g/cm³ |
| Solubility in water | Soluble in water |
| log P | 0.02 |
| Acidity (pKa) | 6.0 |
| Basicity (pKb) | pKb ≈ 8.8 |
| Refractive index (nD) | 1.597 |
| Dipole moment | 4.61 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 274.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -579.6 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P273, P280, P305+P351+P338, P337+P313 |
| Flash point | > 111.5 °C |
| LD50 (median dose) | LD50 (mouse) oral: 320 mg/kg |
| NIOSH | SW4150000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 175 mg/kg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
2-Aminothiazole Thiazole 2-Aminothiazole-4-carboxylic acid Ethyl 2-aminothiazole-4-carboxylate Methyl thiazole-4-carboxylate Methyl 2-bromothiazole-4-carboxylate Methyl 2-amino-5-methylthiazole-4-carboxylate |