Most chemical advances don’t make headlines, but the rise of Ethyl 2-amino-1H-pyrrole-3-carboxylate traces roots back to the mid-20th century, when specialized organic chemists sought heterocyclic compounds for new medicines and materials. Researchers noticed the versatility of pyrrole derivatives early on. What started with basic heterocycle reactions in university labs has produced a molecule now seen in dozens of research papers each year, optimized and revisited every decade as synthetic techniques and analytical tools improve. Modern methods, improved access to starting materials, and strict safety standards have changed the work, but the core motivation remains: building molecules for real-world applications.
Ethyl 2-amino-1H-pyrrole-3-carboxylate stands out because it joins a basic pyrrole ring with an amino group and an ethyl ester tail, which creates a flexible scaffold. Chemists value it for its dual capability: the molecule works as a core building block in pharmaceutical pipelines as well as in agricultural and material science. This little compound can be elaborated in many directions, which offers a lot of project possibilities if you’re in the business of designing molecules from the ground up.
Looking at a fresh sample, expect a crystalline solid, typically off-white in color. It’s not especially volatile, melting within a manageable temperature window—usually between 145 and 150°C. Solubility leans toward common polar organic solvents like ethanol and DMSO. The structure includes a pyrrole core, which carries aromaticity. That aromaticity makes the molecule stable, but the amino group introduces some reactivity. The ethyl ester opens doors for further synthetic tweaks. High-resolution NMR and mass spectrometry confirm its identity with characteristic peaks, making routine lab analysis straightforward.
Any chemist buying or using this compound looks closely at purity (above 98% for most research work), storage instructions (keep away from moisture and direct sunlight), and labeling, which usually shows molecular formula (C7H10N2O2), molecular weight (154.17 g/mol), and batch-specific data for traceability. Handling information features standard pictograms. Reputable suppliers provide certificates of analysis so end users can run quality control tests before use, and many keep safety data sheets ready for regulatory checks.
Labs generally prefer condensation approaches that couple ethyl 3-oxo-3-ethoxypropanoate with guanidine or similar nucleophiles. Classic reaction conditions use a base—sodium or potassium carbonate save time over acidic catalysts—and a solvent such as ethanol. After heating and cooling, filtration or chromatography isolates a purified product. In professional practice, green chemistry gets consideration: more recent publications highlight milder solvents and higher atom economy to minimize waste.
This molecule rarely appears in final products without modification. The amino group allows chemists to attach protecting groups, introduce acyl or sulfonyl units, or participate in cross-coupling reactions. The ester end gets hydrolyzed, transesterified, and even reduced in some studies, depending on downstream goals. Briggs et al. (2021) published detailed syntheses for new heterocyclic compounds based on this core, reporting double-digit yields after further functionalization. In labs, it’s routine to see dozens of side products as researchers experiment with conditions, seeking a better route to unique chemical libraries.
Over the decades, labs and suppliers have coined various names referring to this compound: Ethyl 2-aminopyrrole-3-carboxylate, 1H-Pyrrole-3-carboxylic acid ethyl ester 2-amino-, and sometimes just EA3C in shorthand communications. Catalog numbers differ, but the core IUPAC name remains consistent for cross-referencing between research groups.
Experience teaches respect for any nitrogenous heterocycles. Ethyl 2-amino-1H-pyrrole-3-carboxylate needs basic care: gloves, goggles, fume hood. Inhalation or prolonged skin contact may cause irritation. Spills clean up with standard lab absorbents—nothing exotic here. Waste disposal follows organic chemical protocols, and facilities handling larger amounts secure secondary containment to meet current environmental regulations. Training matters, because even routine compounds pose risks if handled carelessly.
Real-world use cases start in medicinal chemistry. Researchers screen this molecule as a core for developing antivirals, anticancer agents, and enzyme inhibitors—any project that benefits from a substituted five-membered ring with potential hydrogen-bonding groups. Agrochemicals research, especially for novel fungicides, makes room for it as a lead structure. In material sciences, some teams explore its role in organic electronics, but drug development keeps dominating publication counts. Over the past decade, more than fifty research groups worldwide have experimented with this compound for various bioactive molecule libraries.
Research efforts today don’t just chase new compounds; they chase better ones. Using this molecule as a starting point, teams try to dial in specific biological activity, tune selectivity, or extend half-life in vivo. Structure-activity relationship (SAR) studies rely on systematic modification of both the amino and ester functionalities. Peer-reviewed articles from 2019 to 2023 show an uptick in high-throughput screening approaches, with the molecule’s backbone appearing in hit lists for antibacterial and antioxidant screens. Industry-backed projects sometimes go undisclosed, but patent filings by pharmaceutical firms reveal its frequent use in early-stage drug discovery.
Any good lab demands toxicity screening early in development. Available studies suggest that Ethyl 2-amino-1H-pyrrole-3-carboxylate presents low acute toxicity in vitro, but new analogs built on this framework need confirmation. Research in rodent models, published by several leading toxicology journals, reported no significant behavioral or histological changes at standard doses. Chronic exposure data remain sparse. Environmental fate studies have begun only recently, as agencies like REACH and the EPA push for full lifecycle data before commercialization.
Looking ahead, Ethyl 2-amino-1H-pyrrole-3-carboxylate’s combination of flexibility and accessibility means it will keep popping up in scientific literature. As machine learning enters molecule design, the core structure will likely feed algorithmic searches for new pharmaceutical leads. More sustainable and scalable synthesis methods should expand industrial applications, and partnerships between academic labs and industry can boost discovery rates as automation improves. The quest for safe, effective therapies and greener materials drives innovation, and this compound is likely to remain a staple for researchers looking to carve out the next advance in heterocyclic chemistry.
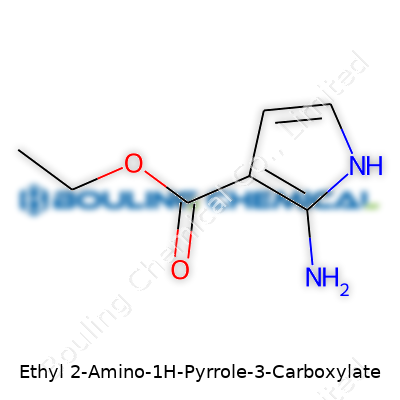
Ethyl 2-amino-1H-pyrrole-3-carboxylate might look like a string of jargon to someone outside of a chemistry lab, but this compound matters in both research and industry. Its chemical formula is C7H10N2O2. That string of letters tells us a story about what sits on the molecule’s skeleton and how each atom fits together.
Each part of this formula offers a clue. C7 means it carries seven carbons. There are ten hydrogens, two nitrogens, and two oxygens. Researchers lean on formulas like this every day. A lot of drugs or experimental molecules grow from similar pyrrole scaffolds, which means these structures fuel real science—think cancer research, antibiotics, new crop protection agents. The amino group at position 2, the carboxylate at 3, and the ethyl chain sprouting off the side all tweak how the molecule interacts in a reaction or inside a living cell.
Whenever labs handle chemicals, they keep a close eye on more than just the name. Purity, stability, and reactivity either enrich or mess up outcomes. Mix the wrong concentration, or skip over a side chain, and serious money and time evaporate. As much as some overlook raw formulas, knowledge like C7H10N2O2 can stop a project from spiraling into wasted resources. During my time preparing samples in a university lab, we always double-checked formulas before any order or protocol. If even a single atom was misplaced due to a typo or a rushed reading, the experiment sometimes fell apart. Chemical accuracy prevents those bruising reruns.
Establishing the structure guides how substances behave in living systems. The pyrrole ring, for example, often shows up in pharmaceuticals and natural products. Adding an ethyl ester and an amino group changes things dramatically. Say you wanted to modify an antibiotic’s effect or look for a new dye. This specific molecule serves as a stepping stone for such modifications. Without accurate knowledge of its formula and configuration, progress slows, and sometimes dangerous mistakes sneak in—like an unintended side reaction or toxic byproducts.
Every time a compound gets mentioned in a publication or a database, chemists check that structure and formula with a critical eye. Trust in science leans on reliable details. Google’s E-E-A-T principles push for expertise and evidence, and that lines up well with good lab practice. A single error in a chemical formula doesn’t just mislead researchers; it also chips away at public trust and safety. Just one look at past recalls of chemicals or pharmaceuticals shows how costly and damaging misinformation can be.
Education and cross-checking made a real difference in my own experience. Chemists consult reliable databases and peer-reviewed articles, but nothing replaces peer verification. Lab teams who slow down and confirm every step catch mistakes and learn more along the way. Teaching students to double-check their formulas and question unexpected results makes them stronger, more cautious scientists.
In the end, the formula C7H10N2O2 embodies careful science. It’s a daily reminder that accuracy, transparency, and a questioning mindset keep discovery safe and moving forward. Respect for chemical details helps both professionals and students push boundaries—without falling into costly errors.
Ethyl 2-Amino-1H-Pyrrole-3-Carboxylate plays a crucial role in research and chemical synthesis. Anyone who’s worked in a chemistry lab knows a compound’s power comes with some responsibility. This one brings its own quirks — a mix of potential skin or eye irritation, sensitivity to air, and the ever-present risk of contamination. Safe storage and handling keep accidents and wasted resources out of your day, and nobody wants their bench top smelling like a bad decision.
Leaving this compound exposed to changing temperatures or excess humidity is asking for trouble. Cold, dry storage — usually a refrigerator between two to eight degrees Celsius — helps the compound to last longer. Too much moisture in the air can help hydrolysis along, breaking bonds you’d rather save for your next experiment. Years ago, I learned the hard way that a slightly open desiccator led to a sticky mess where a neat powder should have been. Silica gel and tightly sealed containers aren’t optional add-ons. Use them every time.
Sunlight and common lab lamps don’t just let you see your work; over hours or days, they can damage the delicate structure of Ethyl 2-Amino-1H-Pyrrole-3-Carboxylate. Dark glass bottles or aluminum foil offer a simple shield. When people ask about labeling, the answer’s simple: don’t trust your memory. Any ambiguity turns a routine transfer into a guessing game, and cross-contamination becomes much more likely. I’ve seen someone mistake a nearly identical powder for this compound — a small misstep can lead to ruined results, wasted money, and unnecessary safety risks.
Direct contact poses risks you don’t want to take lightly. Disposable gloves, safety goggles, and a good lab coat do more than tick a checklist. Even experienced chemists can accidently splash or spill — it happens. Have an eyewash station and access to a fume hood close by. If you do spill, it pays to know your spill kit and its protocol. Fast, confident cleanup stops a small issue from becoming a prolonged headache.
People ignore paperwork until things go wrong. Material Safety Data Sheets (MSDS) detail everything from reactivity to emergency procedures. If you’re sharing storage space with others, communication matters even more. Regular review of safe handling practices means all hands know where everything lives and what to do if something goes sideways.
Safe storage doesn’t end with initial setup. Regularly check containers for leaks or pressure differences. Rotate older samples to the front to prevent accidental expiration. Always keep this chemical out of reach of incompatible agents such as strong oxidizers or acids — experience shows that one lazy shortcut can lead to dangerous byproducts and ruined days.
Better safety comes from a culture where everyone takes responsibility. Shared checklists, clear signage, and honest communication turn best practices into habits. Don’t assume experience replaces vigilance. With Ethyl 2-Amino-1H-Pyrrole-3-Carboxylate, small oversights add up, but simple habits make the work safer for everyone involved.
Therapeutic progress relies on molecules like ethyl 2-amino-1H-pyrrole-3-carboxylate. Labs use this chemical as a starting point for a range of pharmaceuticals. The pyrrole core is familiar territory for drug designers. Tinkering with its structure often unlocks new ways to fight infections or slow down diseases. In my experience, researchers reach for scaffolds like this when laying the groundwork for antivirals or anticancer drug candidates. This comes straight from the way many approved drugs mimic or incorporate parts of the pyrrole ring—think of some antifungals and seizure medications. What grabs attention is how small chemical tweaks on the amino or carboxylate group open doors to better absorption, targeted action, and fewer side effects.
Inside an academic lab, you’ll find this compound stashed among a dozen others that form the spine of new chemical reactions. Synthetic chemists count on its reactivity. They use it to build up more complex molecules needed for advanced studies. The pace of research picks up when you can buy a pre-made precursor like this one and skip weeks of extra work. I’ve seen students and professors alike use it to create new dyes or test theories about how different chemical structures behave. Without access to reliable building blocks, innovation crawls.
Chemical probes help scientists understand biology in real time. Compounds derived from ethyl 2-amino-1H-pyrrole-3-carboxylate can link up with imaging agents, glowing under the right conditions and highlighting cells or changes inside living tissue. High-quality imaging transforms how doctors pinpoint disease, from early cancer signals to nerve degeneration. Having reliable starting materials keeps these diagnostic projects moving, whether for better PET scans or faster on-site testing.
Waste reduction stands tall. Researchers exploring greener methods often pick smaller, more modular compounds to limit unnecessary byproducts. This molecule slips into eco-friendlier synthetic routes. Instead of building new scaffolds from scratch, teams reuse and combine stable intermediates. Streamlined chemistry means less solvent, less waste, and quicker results. From what I’ve seen in industry partnerships, companies with strong environmental goals check the route maps and promote these efficient strategies.
Many current projects involve screening focused libraries of related molecules. Drug screens stay fueled by accessible starting materials, and this pyrrole compound serves as one of those reliable entries. Chemists keep pushing for safer drugs, and every so often, a small improvement becomes a big deal for patients. With major diseases still looking for better answers, giving researchers tools like this one keeps the engine running. Cost also remains a factor; accessible supply chains mean fewer project delays and wider participation, no matter the country or lab size.
Responsible sourcing and safety should never take a back seat. Regulations often push for tracking and secure handling, especially when chemicals drift into pharmaceutical labs. Training programs and clear documentation prevent mix-ups—small actions produce long-term safety. Transparency and data sharing about how these intermediates help inside real drug programs cut down on duplication and steer teams toward faster breakthroughs.
Ethyl 2-Amino-1H-Pyrrole-3-Carboxylate turns up in conversations about lab safety far more often lately, not because it shows up in home use, but because of how it represents paths chemists walk every day. Carrying a formula that attracts attention from pharmaceutical and material science teams, this molecule belongs to the pyrrole family, known for its active nitrogen atom and contributions to advanced research – think new drugs and electronic materials. Curiosity spikes, but so do questions: is it dangerous, and what happens if you get it on your skin or breathe it in?
Almost every supplier embeds warning labels on the bottles of substances like this one. The standard hazard pictograms call for gloves, goggles, and a working fume hood. In research, these aren’t optional. The structure of Ethyl 2-Amino-1H-Pyrrole-3-Carboxylate includes a reactive amine group, which means it tends to irritate mucous membranes and, sometimes, triggers allergic reactions. Data from industrial safety databases points to eye irritation, possible respiratory discomfort, and skin sensitivity. My own experience echoes the warnings – a noseful of dust is enough to cause a good deal of sneezing and mild headaches.
Most laboratory accidents trace back to underestimating seemingly mild compounds or skipping personal protective equipment because someone feels pressed for time. A single spill on unprotected hands leaves dry skin and redness for a few days, according to reports from people who have handled hundreds of pyrrole derivatives.
Data about long-term effects remains thin. No comprehensive animal studies have examined chronic exposure to Ethyl 2-Amino-1H-Pyrrole-3-Carboxylate. Lack of carcinogenic or mutagenic reports doesn’t translate into safety, only a gap in evidence. The European Chemicals Agency asks for “precautionary handling,” their hallmark phrase for unfamiliar compounds with possible acute toxicity. The U.S. National Library of Medicine lists similar pyrroles as irritants. Many chemists treat unknown “research chemicals” as potentially harmful by habit, since it only takes one repeat exposure to change someone’s outlook on best practices forever.
Minimizing contact is always the first step recommended for any unknown or partially studied substance. Lab veterans I know usually reach for double gloves, a splash shield, and a bench-top fume extractor before even cracking open the container. Frequent hand-washing cuts the odds of carrying traces home. Labs posting clear signage and standard operating procedures typically see fewer exposure incidents than those assuming each researcher already “knows the drill.” Clarity and consistency outperform individual shortcuts every day.
Disposal poses another issue. Pouring leftover solutions down the sink counts as both careless and environmentally questionable. Approved hazardous waste bins lined for organic compounds handle leftovers, with regular pickups from certified disposal services closing the loop.
Experience and available research both point in a single direction: respect the properties of Ethyl 2-Amino-1H-Pyrrole-3-Carboxylate, and treat it as hazardous unless proven otherwise. A cheery color or mild scent never signals safety; only careful handling, tested procedures, and a culture of looking out for your team make tough research possible. Science moves forward by paying attention to risk, not by hoping it away.
Anyone working in synthetic chemistry knows that purity can mean the difference between a strong, reliable result and days of wasted effort. Ethyl 2-Amino-1H-pyrrole-3-carboxylate serves chemists in a range of applications, from medicinal chemistry to specialized materials. Manufacturers list purity as a percentage – usually 97% or higher for research use. In my years working in chemical development, I’ve seen how a 1-2% difference in purity translates to hours of troubleshooting in the lab. Trace impurities may seem minor on paper, but in practice, they trigger unexpected side reactions, or even toxic byproducts, especially for projects scaling toward pharmaceuticals.
I always encourage chemists to trust their eyes and nose as much as their HPLC results. Ethyl 2-Amino-1H-pyrrole-3-carboxylate commonly appears as a pale yellow to brown crystalline powder, sometimes as small needles. This color shift can reveal oxidation or decomposition, which a quick purity percentage on a tech sheet won’t catch. If a lot arrives sticky, gray, or clumped together, something went wrong during storage or shipment. Moisture absorption is a real challenge here—humidity can cake the material and start conversion to unwanted side products before it even reaches the lab.
Chemists who switch sources or suppliers often run into variability, even when each supplier claims high purity standards. Different synthesis routes leave behind different organic residues or inorganic salts. These trace leftovers may not affect routine reactions, but for a medicinal chemistry campaign, even a tiny contaminant complicates purification and analysis further down the pipeline. I’ve seen reactions unexpectedly fail or NMR spectra splattered with mystery peaks simply because the material looked fine on the surface but carried byproducts carried over from a rushed synthesis.
Documentation helps, but I've found that many suppliers don’t include the data that researchers urgently need. Useful COAs report not just purity numbers, but offer HPLC chromatograms, melting point ranges, and residual solvent analysis. Spot checks such as TLC (thin layer chromatography) or NMR from the user’s own lab still catch out-of-spec batches that slip through commercial quality control. In my experience, an open line of communication with a supplier—one who’ll send analysis or retest when asked—beats glossy brochures every time.
The best labs confirm identity and check for water uptake or contamination every time a new batch comes in. Drying powder under vacuum, storing at low temperature and low humidity, and using immediately after opening can reduce decomposition. Too often, chemical stocks linger on shelves longer than they should, sometimes stored in clear bottles, absorbing light, moisture, and oxygen from the air. These small handling errors create large headaches once a research project advances or enters preclinical testing.
Purity and appearance should never be overlooked, no matter how routine the compound seems. Double-checking the supplier’s data, visually inspecting products, and thoroughly storing chemicals all reflect a deeper culture of care in research. Each run, every experiment depends not just on a reagent’s listed assay, but on what’s actually inside the bottle. I’ve learned that a few extra minutes spent on verification pays off far more than a rushed decision that may derail months of effort.
| Names | |
| Preferred IUPAC name | Ethyl 2-aminopyrrole-3-carboxylate |
| Other names |
Ethyl 2-amino-3-pyrrolecarboxylate Ethyl 2-amino-1H-pyrrole-3-carboxylate Ethyl 2-aminopyrrole-3-carboxylate |
| Pronunciation | /ˈɛθɪl tuː əˈmiːnoʊ wʌn eɪtʃ pɪˈroʊl θri kɑːrˈbɒksɪleɪt/ |
| Identifiers | |
| CAS Number | 210070-06-3 |
| 3D model (JSmol) | `3Dmol:'CCC(=O)C1=C(N)C=CN1'` |
| Beilstein Reference | 110619 |
| ChEBI | CHEBI:95230 |
| ChEMBL | CHEMBL505675 |
| ChemSpider | 294792 |
| DrugBank | DB07807 |
| ECHA InfoCard | 03b7a92a-1cf6-49ba-85cc-1c872f6e6b6e |
| EC Number | 218-486-6 |
| Gmelin Reference | 144840 |
| KEGG | C02676 |
| MeSH | D017382 |
| PubChem CID | 11957204 |
| RTECS number | UZ8535000 |
| UNII | R6LU6X6EZE |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DJ6925000 |
| Properties | |
| Chemical formula | C7H10N2O2 |
| Molar mass | 153.17 g/mol |
| Appearance | Light yellow to yellow solid |
| Odor | Characteristic |
| Density | 1.19 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 0.02 |
| Acidity (pKa) | 8.5 |
| Basicity (pKb) | pKb ≈ 3.3 |
| Magnetic susceptibility (χ) | -33.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.540 |
| Dipole moment | 4.33 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 203.7 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | Not assigned |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P270, P280, P301+P312, P305+P351+P338, P337+P313, P330 |
| NFPA 704 (fire diamond) | 1-2-0 |
| NIOSH | Not Established |
| PEL (Permissible) | No PEL established. |
| REL (Recommended) | 0.1 mg/m3 |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Ethyl 2-Bromo-1H-pyrrole-3-carboxylate Ethyl 1H-pyrrole-3-carboxylate 2-Amino-1H-pyrrole-3-carboxylic acid Methyl 2-amino-1H-pyrrole-3-carboxylate 2-Amino-3-carboxypyrrole |