Dimethoxymethane, sometimes called methylal, traces its use back to the late 1800s. Chemists first looked at it as a possible alternative to ether. Early scientific studies noticed how the compound’s volatility and solvent power could play a role beyond the lab. Over decades, increased understanding of its chemistry grew out of industrial needs like cleaning fluids and fuel additives. Production scaled up through the twentieth century, matching new requirements across sectors including automotive and coatings. Laboratory work continually shed light on the best ways to create and handle this solvent safely, while industry standards shifted to reflect new findings about flammability and toxicity.
Dimethoxymethane is a highly flammable, colorless liquid with a mild, ether-like odor. It offers low viscosity and a boiling point near 42°C, very close to diethyl ether. You’ll often see it used where rapid evaporation and solvent strength matter more than heavy solvency. Methylal dissolves in a range of organic compounds, making it handy for quick cleaning and as a blending solvent in paints and coatings. Its clean-burning nature also led to attention from fuel formulators looking to boost octane or improve cold starts. Some smaller-scale uses include cosmetic formulations and as a medium for chemical reactions demanding little moisture.
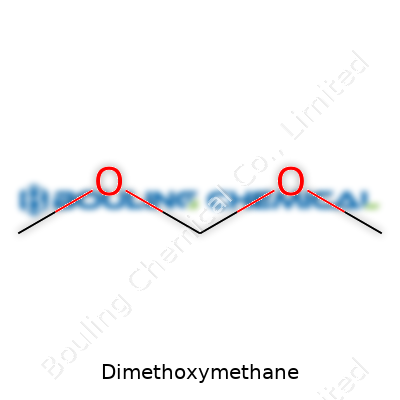
The molecular formula reads CH3OCH2OCH3, pointing toward a diether with a low molecular weight of just 76.09 g/mol. Compared to other ethers, Dimethoxymethane evaporates quickly thanks to its high volatility and low boiling point. It weighs less than water, with a specific gravity around 0.86 at room temperature. The compound mixes well with alcohols, ethers, esters, and hydrocarbons but not with water. It burns cleanly, producing water and carbon dioxide, displaying its stability under mild conditions but increasing risks in hot or open environments. Chemical testers measure a flash point around -17°C, so fires can spread quickly unless strict control standards stay in place.
Labeling on containers often reads: Dimethoxymethane, Methylal, CAS No. 109-87-5, UN No. 1230. Tech data sheets from producers will list purity, usually above 99%, and may show residual water content, acidity, and specific gravity. Packaging for industry aligns with flammable standards—tanks and drums come marked with “Flammable Liquid” warnings and storage advisories calling for cool, ventilated spaces away from open flames. Occupational Safety and Health Administration (OSHA) and similar bodies expect clear secondary labeling for any transfer outside original drums, along with spill protocol instructions.
Most industrial processes create Dimethoxymethane by reacting excess methanol with formaldehyde using both acid and base catalysts under mild heat. Controls for temperature and pH mean batches yield methylal with low impurities. The industry prefers continuous reactors to avoid batch variation and trim energy costs. Catalyst regeneration and the use of energy-efficient distillation shape today’s greener production efforts. Handling of formaldehyde and methanol always demands air controls, scrubbing, and careful waste management to protect workers and the environment.
Dimethoxymethane takes part in different chemical syntheses. As an acetal, it provides a source of the methoxymethyl group for protecting alcohols during organic synthesis. In fuel chemistry, it reacts with certain fuels and additives, and under strong acid, can hydrolyze back to methanol and formaldehyde, which reinforces the need for precise storage. Its reactivity means it can’t be left in acidic conditions for long or combined with oxidizing agents; runaway heat or pressure can occur. In the lab, researchers look at how derivatives of methylal might help develop new solvents or even polymers, reflecting a wider chemical interest in simple ethers as building blocks.
Industry, academia, and commerce know this chemical by several names: Dimethoxymethane, methylal, formaldehyde dimethyl acetal, and dimethyl formal. Brand formulations sometimes put the methylal tag in the product name, or mention its use in fuel blends or cleaning solutions. Safety data sheets will pin down the synonyms and regulatory identifiers, helping everyone along the supply chain recognize the hazards and best uses without confusion.
Safety work around Dimethoxymethane starts with recognizing fire risk. Facility storage fire codes require flame arrestors, grounding, and sealed containers. Good ventilation in rooms and any transfer area helps prevent vapor buildup, addressing health and explosion concerns. Personal protective gear—gloves, goggles, and flame-resistant clothing—remains standard. Spills demand immediate clean-up with non-sparking tools and chemical absorbents. OSHA exposure limits protect against headaches, dizziness, or longer-term nervous system damage. In every workplace, clear training and emergency plans ensure readiness, since mistakes can have serious consequences in confined or busy industrial settings.
Dimethoxymethane’s largest uses show up in the paint and coatings field, where its rapid evaporation speeds up drying. It works in cleaning agents for electronics, engine parts, and delicate instruments. In fuels, methylal acts as an oxygenate to raise octane and reduce emissions—China and other regions continue to look into it for gasoline formulations, although blending challenges and volatility limit widespread adoption. Laboratories use it to protect reactive alcohols in complex syntheses. Personal experience with methylal includes its value in the clean-up of precision optical parts, where residue-free evaporation beats water-based alternatives. Research institutions sometimes push Dimethoxymethane into new roles such as solvent for battery electrolytes, chasing improvements in charge-discharge rates or safety profiles.
Researchers dig deeper into Dimethoxymethane’s performance as a sustainable fuel component. Big attention goes toward its potential in reducing greenhouse emissions compared to conventional gasoline, especially in regions focused on cutting smog. Ongoing work targets safer manufacturing, such as greener catalyst choices and energy-cutting production loops. Scientists seek to modify methylal for new drug intermediate steps or as a reaction solvent that cuts out toxic residues present in common alternatives like toluene or dichloromethane. Trials keep looking for ways to pair methylal with battery or polymer science, hoping to inch toward breakthroughs in performance, safety, or recyclability.
Toxicologists chart short- and long-term effects of Dimethoxymethane inhalation. At room temperature, vapor exposure causes dizziness and headache, while high doses can lead to central nervous system depression. Animal studies point to low acute toxicity by oral or dermal exposure, but chronic testing flags repeated vapor inhalation as a risk for mucous membrane irritation or liver strain. The compound’s breakdown products—formaldehyde and methanol—raise big red flags, so proper storage and handling stay non-negotiable. Regulators around the world set occupational exposure limits well below acutely toxic levels, with guidance on ventilation, detection alarms, and rapid response protocols for spills or leaks.
Dimethoxymethane’s outlook hinges on safety, regulatory controls, and innovation. Stricter rules in coatings and fuels force companies to rethink reliance on volatile organics, but methylal’s fast evaporation and clean profile keep it in the running. Companies pursue next-generation formulations with lower toxicity and better environmental footprints, using methylal as a replacement for more hazardous solvents. Interest continues in blending it with advanced fuels, alongside research into how its reactivity might support pharmaceutical and polymer chemistry without bringing new environmental headaches. Future advances will depend on marrying good performance with better safeguards and greener sourcing, letting science and policy shape how Dimethoxymethane gets used in tomorrow’s industries.
Dimethoxymethane, better known to some as methylal, looks clear and smells sweet. People working in labs often keep a bottle close, but its reach goes further. I first noticed its importance during a stint helping at a specialty coatings shop. Before long, I saw it popping up everywhere—solvents, fuel treatments, cleaning mixtures.
Anybody who has opened a can of spray paint has brushed shoulders with dimethoxymethane’s power. Paint companies use it for its ability to dissolve pigments and resins that would otherwise clump or harden too soon. Spraying paint should mean even coverage, quick drying, and a smooth finish. This chemical handles oil, acrylic, and nitrocellulose bases. Factories mix it with ketones or alcohols to create blends that work across temperatures and surfaces. I remember helping an auto body shop switch to a dimethoxymethane-based thinner—the difference in drying times and finish stuck with me, because customers waited less and cars looked better.
Not only painters benefit. This compound finds a home in making fuels burn cleaner. Fuel makers blend it into gasoline and diesel to boost their octane rating. That means more complete combustion, which lowers soot, carbon monoxide, and unburned hydrocarbons pumping out of tailpipes. Dimethoxymethane burns with less smoke, and some research points to it helping cut nitrogen oxide emissions. China and parts of Europe have even tested it as a main ingredient in alternate fuels—an answer to tightening pollution rules and cleaner engine goals.
Factory floors get filthy. Machines need cleaning, especially when grease and oil build up. It’s tempting to reach for harsh stuff, but dimethoxymethane cleans without etching metal or leaving residue. Electronics repair shops use it to wipe away stubborn flux. Some dry cleaners mix it into solutions for spot treatments on delicate fabrics. Its quick evaporation means less risk of lingering damp spots or fumes after a cleaning.
Beyond obvious uses, chemists value dimethoxymethane for making other things. Its structure includes two methoxy groups, making it a handy ingredient in reactions that need mild, predictable conditions. Drug manufacturers sometimes use it to add protective groups during synthesis, controlling where reactions happen on complex molecules. Lab work might seem far from daily life, but these processes turn raw compounds into medicines or specialty polymers.
Nothing comes without drawbacks. I’ve seen workers get lax about ventilation, thinking a little solvent smell means nothing. Dimethoxymethane can irritate skin and eyes or give headaches in high doses. It’s flammable, which makes safe storage and use critical in crowded shops. Regulators in the US, EU, and Asia track how much vapor enters air and water, so factories run testing and install scrubbers. Workers need gloves, masks, and plenty of fresh air to keep exposure below dangerous levels. On a busy shift, it’s easy to skip these steps, but I’ve spoken with too many who regret ignoring those early warning headaches.
Some call for greener, safer solvents and additives as climate rules sharpen. Companies experiment with blends to reduce risks and hang onto dimethoxymethane’s best traits. Closed-loop systems catch vapors for reuse. Training workers to watch for spills and keep records goes a long way. Switching to water-based cleaners or less-flammable ingredients can take time and research, but my own experience with trial and error in small labs shows the right move pays off. Vigilance and respect for this unsung industrial tool help keep its benefits front and center, while risks stay in check.
Most people outside chemistry and industry don’t usually hear about dimethoxymethane. This colorless, flammable liquid finds its way into adhesives, paints, some fuels, and cleaning products. Factories may use it as a solvent or blending agent. Its sweet, ether-like smell gives it away. Yet, for those actually working near the stuff, fear and curiosity about safety turn up with regularity.
Folks sometimes assume every chemical name must mean danger, but evidence should guide these opinions. Short-term inhalation can cause mild dizziness, headache, or throat irritation. Dimethoxymethane vapor may also irritate eyes and skin if contact happens often or in large amounts. Health concerns mostly show up in workspaces where ventilation falls short or personal protection isn’t taken seriously.
Animal tests on high-dose exposure over several hours show effects on the central nervous system. Still, these doses go far beyond what the average worker encounters with reasonable controls. No major governing health agency has yet found enough evidence to list this chemical as a cancer risk for humans.
Companies relying on dimethoxymethane usually follow guidance from sources like the US Occupational Safety and Health Administration (OSHA) or the European Chemicals Agency. The law calls for protective gloves, splash goggles, and good air systems. Workers know the routine: keep skin covered, avoid breathing mist, and don’t eat or drink near the stuff. A mask and fan work wonders.
On the few occasions spills or accidental splashes occur, the industry standard is to leave the area, find plenty of fresh air, and rinse skin or eyes straight away. Using soap—never solvents—matters. Supervisors hold regular safety briefings because new hires and careless veterans both need reminders.
For most folks shopping at the hardware store, dimethoxymethane in an aerosol or adhesive comes heavily diluted. Bottles contain safety warnings, but simply breathing near a recently glued item won’t match the intense exposure seen in warehouses or factories. Open windows or fans sweep away low-level vapors. Still, those with asthma or allergies sometimes react, though complaints stay mild and fade away quickly.
The big players in chemical safety work hard to cut down risk. Substituting dimethoxymethane with less volatile or less irritating chemicals, whenever possible, keeps health complaints down. Training does more than any warning label ever could: people remember a real story over a poster. Keeping Material Safety Data Sheets nearby makes sense, offering quick facts if something goes wrong.
Emergency kits with clean water, gloves, and extra masks make a difference on the floor. Management setting a culture that values speaking up about spills or bad smells means less guesswork and more trust. If dimethoxymethane starts causing repeated or strange effects among staff, workplaces bring in outside experts or health inspectors who can run air tests and dig into records. It beats waiting for a major incident.
Chemicals like dimethoxymethane come with risk—so the answer remains about respect, not fear. Routine, knowledge, and good training offer real protection, both for people making a living and for everyone at home using store-bought products.
Storage might not seem exciting, but it shapes daily work for anyone in the chemical field. Dimethoxymethane pops up across labs and industry, and keeping it safe is about more than just following the rules on a safety data sheet. It boils at a low temperature and catches fire easily, so one wrong move brings risk. Just a cracked lid or leaky valve can fill a room with its sweet, ether-like odor. Inhaling too much for even a short time feels rough. Storage methods that prioritize health, safety, and fire prevention aren’t just legal formalities—they protect real people who work with this chemical.
This isn’t a chemical that likes heat or open spaces. Dimethoxymethane evaporates fast and forms flammable vapor at typical room temperature. Its flash point hovers below freezing, which means it can light up before folks realize danger is building. Its vapors are heavier than air and can pool low to the ground, slipping along floors toward ignition sources without much warning. That’s why it pays to choose a storage spot where temperatures stay stable and cool, and where good ventilation moves any vapors outdoors instead of letting them gather.
Smart storage starts with the right container. Metal drums with tight-fitting lids stand up well against chemical leaks and block sunlight, which can break this solvent down over time. Glass works for small lab-scale bottles, but not in places with a lot of movement. Seals should always be checked—synthetic materials like fluoropolymer liners hold up better than basic rubber, since dimethoxymethane can slowly eat away at less hardy stoppers. Clear, permanent labeling guards against rushed mistakes in busy labs and storage rooms.
A moment’s carelessness can cost dearly. Any spark, whether from static, a flicked switch, or nearby welding, spells trouble where dimethoxymethane sits. Store away from open flames, heating elements, and hot piping. Electrical equipment inside a storage space should meet the standards for flammable atmospheres—no basic light fixtures or sockets. Spill control means keeping absorbents, spill kits, and extinguishers ready at the door. Simpler steps—like grounding metal storage shelves to discharge static electricity—make a surprising difference. The best fire prevention isn’t flashy; it sits in small daily habits, like walking a bottle back to the shelf instead of leaving it on a bench.
No one should grab a bottle of dimethoxymethane without knowing what they're handling. Training for everyone with access—briefing on risks, spill cleanup, and first aid—cuts down on accidents. Some places use locked chemical cabinets, with logs or digital tracking for every check-in and check-out. Storing only the needed amount, rather than overstocking, limits risk. Rotating stock so the oldest containers are used up first avoids forgotten, deteriorating bottles that could leak or burst.
Leaking dimethoxymethane doesn’t just threaten people indoors. Without careful handling, it seeps into soil and water. Drains are out of the question, and careless dumping brings trouble far beyond the storage room. Partnering with certified disposal companies and following local hazardous waste guidelines closes the loop, providing accountability that stretches from delivery to disposal.
Storing dimethoxymethane isn't about ticking boxes—it's about protecting everyone from the unseen hazards waiting in a flammable vapor or a cracked lid. Following best practices, storing in the right conditions, choosing strong containers, and keeping vigilant about access all work together. Everyone carrying a bottle, opening a cabinet, or checking stock plays a role in keeping people and the environment safe.
Dimethoxymethane won’t usually pop up in daily conversation, but in the world of chemistry, it gets plenty of attention. Chemists know it as CH3OCH2OCH3. You can also see it written as C3H8O2. The name gives a hint about its structure: two methoxy groups linking up with a methylene bridge.
Working in a university chemistry lab as a student, I came across this compound while cleaning up glassware after an experiment that left everything smelling faintly sweet and ether-like. That smell came from a bottle marked “methylal”—another name for dimethoxymethane. Later, in an applied research project about fuel additives, I saw how methylal gets used for more practical purposes. It pops up in industries where solvents and fuel blending agents help clean up processes or improve engine performance.
Anyone who has ever worked with organic solvents knows the formula isn’t just something to memorize for an exam. The atoms in C3H8O2 line up to create low toxicity, high volatility, and an almost perfume-like odor. These traits help explain why it works in things like paint stripping, plastics processing, and even as a blowing agent for foams.
Chemicals that evaporate quickly and don’t leave behind toxic byproducts serve valuable roles, especially as industry faces pressure to cut down on pollution and worker exposure. Dimethoxymethane, compared to more hazardous ethers, proves to be easier on the environment and the human body, though handling it safely always stays non-negotiable—direct contact or inhalation in a closed space is never a bright idea.
It’s easy to get carried away with the benefits and forget the obvious risks. Working with liquids that vaporize fast brings fire hazards. I remember reading reports from local fire departments about factory fires traced to poor ventilation and improper solvent storage. Such cases keep popping up even now, which shows the need for solid safety training on every job site.
Basic steps can prevent most accidents. Decades-old lab safety posters do the job: keep flammable chemicals away from heat sources, use proper containers, and store solvents in a dedicated chemical cabinet. A $200 investment in a decent ventilation fan or explosion-proof refrigerator can save much bigger headaches down the road. Industry groups like the American Chemical Society assemble checklists and training videos that help a lot more than dry technical manuals ever could.
Regulations keep tightening as cities update air quality standards and workers demand safer jobs. As a result, companies have to prove that chemicals like dimethoxymethane meet both safety and environmental expectations. Scientists experiment with blending methylal into motor fuels to cut emissions, while others test alternatives in search of even lower toxicity and less smog-forming potential.
Some manufacturers now rely on greener synthesis routes, aiming to cut down on waste and use renewable feedstocks where possible. Academic labs continue to look for the next big breakthrough—a solvent just as effective but sourced from corn or sugarcane instead of oil. Until those options scale up, the chemical makeup and responsible use of compounds like dimethoxymethane deserve full attention, from the classroom to the production floor.
References: - PubChem Database. Dimethoxymethane C3H8O2. - American Chemical Society. Safety guidelines for laboratory solvents. - “Solvents in the Workplace: Risks, Controls, and Safe Practices,” Journal of Chemical Health and Safety, 2021.
Dimethoxymethane shows up in labs and industry as a solvent. People also call it methylal. It's colorless, with a strong, sweet smell, and it catches fire easily. With a flash point below room temperature, just a spark or a hot surface can set it off. I've watched as even experienced workers get comfortable with this compound and skip over critical steps. That's when accidents happen.
The usual lab coat and gloves are a start, but it's easy to forget about safety glasses or face shields. Years ago, I saw a technician cleaning glassware splash a small amount in his eye—he had skipped his goggles for just a quick rinse. That led to a rushed trip to eyewash and a lost afternoon. Proper gloves, goggles, and a well-fitted lab coat make a difference every single time.
Dimethoxymethane’s fumes get dangerous quickly. The vapor is heavier than air and can build up near floors. In one lab, a technician assumed an open window would do the job. The fumes still crept along the bench, leading to dizziness and a forced evacuation. Always work under a chemical fume hood. Test the airflow. Don’t store open containers outside of the hood. General room fans won’t cut it; vapors just drift and hang around. Make sure the extraction system runs during any use, not just for large-volume transfers.
Leaving a bottle of dimethoxymethane uncovered, even for a moment, fills the room with a flammable mist. Static electricity gives enough energy to spark a fire. Switch off sources of ignition before opening containers. Handle with tools that prevent static build-up. Even cell phones can spark in the wrong situation. Keep firefighting gear rated for chemical fires close—not in a locked cabinet down the hall. Train everyone in the lab so nobody hesitates if something catches.
If a container tips, act right away. Absorb spilt liquid with inert material such as sand or vermiculite. Bag it in proper waste containers, and never flush it. Open all windows and doors, but don’t use regular fans; you can push the vapors into places you don’t want them. Use PPE throughout cleanup. Even small spills can cause headaches and nausea. Call in trained responders for big leaks.
Store it in well-sealed, labeled bottles away from sunlight and heat. Even short exposure to warmth speeds evaporation, and vapors creep into the rest of the work area. Stash bottles in flammables cabinets, never on open shelves. Double-check labels every week. A faded or missing label led to an incident in my college lab where someone poured the wrong liquid into a reaction and had to evacuate the building. It takes five seconds to relabel, but hours to clear out after an unnecessary scare.
Everyone who steps into a lab or workspace should know about these risks and safe routines. Emergency procedures should not stay on paper; drills make habits. A clear, practical safety culture works best. People look out for each other, not just rules on posters.
| Names | |
| Preferred IUPAC name | Methoxymethoxymethane |
| Other names |
Methylal Methylalcohol formal Dimethoxymethane Methoxymethane Formaldehyde dimethyl acetal |
| Pronunciation | /daɪˌmɛθ.ɒk.siˈmiːˌθeɪn/ |
| Identifiers | |
| CAS Number | 109-87-5 |
| 3D model (JSmol) | `/cgi-bin/jmol?model=C1OCOC1` |
| Beilstein Reference | 1361111 |
| ChEBI | CHEBI:42250 |
| ChEMBL | CHEMBL14242 |
| ChemSpider | 7494 |
| DrugBank | DB03755 |
| ECHA InfoCard | DTXSID9020718 |
| EC Number | 203-714-2 |
| Gmelin Reference | 68284 |
| KEGG | C01383 |
| MeSH | D008939 |
| PubChem CID | 11229 |
| RTECS number | PA3325000 |
| UNII | 6UAP4Q7D5N |
| UN number | UN1165 |
| CompTox Dashboard (EPA) | DTXSID2020242 |
| Properties | |
| Chemical formula | C3H8O2 |
| Molar mass | 76.094 g/mol |
| Appearance | Colorless liquid |
| Odor | Pleasant, ether-like |
| Density | 0.861 g/mL at 20 °C |
| Solubility in water | miscible |
| log P | 0.15 |
| Vapor pressure | 669 mmHg (20 °C) |
| Acidity (pKa) | 20.2 |
| Basicity (pKb) | 0.35 |
| Magnetic susceptibility (χ) | -49.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.344 |
| Viscosity | 0.45 mPa·s (20 °C) |
| Dipole moment | 1.30 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 265.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -393.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1605 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H319, H336 |
| Precautionary statements | Precautionary statements: P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P304+P340, P312, P337+P313, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) | 1-4-0 |
| Flash point | -17 °C |
| Autoignition temperature | 224 °C |
| Explosive limits | 3.5–23.5% |
| Lethal dose or concentration | LD50 oral rat 5,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 5650 mg/kg |
| NIOSH | QJ0525000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Dimethoxymethane: "1000 ppm (3100 mg/m³) TWA |
| REL (Recommended) | 12 |
| IDLH (Immediate danger) | 3000 ppm |
| Related compounds | |
| Related compounds |
Methoxymethanol Formaldehyde Methanol Dimethyl ether |