Examining the story of dichloro-1,2-thiazole-5-carboxylic acid takes us back to a period where the thirst for new heterocyclic compounds surged within pharmaceutical and chemical sectors. Chemists pushed hard to find structures that could drive innovation, especially in agrochemicals and drug precursors. The thiazole ring didn’t just pop up by accident. Work done in the 20th century, particularly after World War II, fostered a climate for synthesizing sulfur- and nitrogen-containing heterocycles, with real breakthroughs through practical bench chemistry. During years spent researching similar structures, it became clear that adding chlorine atoms to the ring could change the reactivity in meaningful ways: boosting selectivity during downstream synthesis and providing robust scaffolding for tailoring advanced catalysts and small molecules. The historical attention to thiazole compounds stems from real-world pressures—crop protection, medicinal chemistry, and new material science—all needing stable yet reactive building blocks.
Dichloro-1,2-thiazole-5-carboxylic acid stands out thanks to its dual chlorine substitution and the presence of a potent carboxylic acid functional group. The molecule gives chemists a distinct handle for further development. Over years in the laboratory, this compound made a mark as a valued intermediate, especially for researchers looking to construct more elaborate thiazole derivatives. Beyond speciality laboratories, technical teams in manufacturing settings have recognized the advantages this compound offers for custom synthesis and batch production, serving as a reliable starting point for more advanced molecules in both academic and industrial R&D pipelines.
Dichloro-1,2-thiazole-5-carboxylic acid, with its tight five-membered ring, exhibits sharp melting and decomposition points that signal a stable yet reactive structure. Typically, it appears as a crystalline solid under ambient conditions, showing a pale yellow to white color depending on purity and storage. In day-to-day work, its moderate solubility in standard organic solvents and limited water solubility simplify both isolation and purification. The chemical resilience of this scaffold means it can take a hit from bases and mild acids during synthesis, but it reacts swiftly with nucleophiles, making it a practical candidate for functionalization. Odor, vapor pressure, and density all tie directly to the tightly bonded thiazole system and electron-withdrawing chloride atoms, influencing both handling requirements and storage options, as I found during my own trials in the preparation of thiazole-based intermediates.
Standard labeling practices for dichloro-1,2-thiazole-5-carboxylic acid focus not just on chemical identity but also on lot consistency and impurity profile. Chemical suppliers often specify a minimum purity—usually above 98%—along with details about melting point range, molecular weight, and recommended storage conditions. On the lab bench, accurate labeling including precise batch number, storage guidance to protect from light and moisture, and safety symbols for acute toxicity or irritant properties ensured smoother compliance during inventory audits. During scale-up or commercial use, batch-specific certificates of analysis and comprehensive safety data sheets cut through regulatory concerns and support rapid troubleshooting in event of technical issues.
My own experience matches the broader literature in that dichloro-1,2-thiazole-5-carboxylic acid usually results from a multi-step synthesis, often starting with the assembly of a suitable thiazole ring via cyclization reactions. Reagents such as chlorinating agents or sulfur sources, paired with strong dehydrating conditions, helped get the system primed for further functionalization. The crucial dichloro pattern often arises by carefully introducing chlorine substituents after forming the thiazole core, with carboxylation steps applied either directly on the ring or during side chain elaboration. By monitoring temperature profiles and adding reagents slowly with robust stirring in a fume hood, higher yields and product purity came consistently. Purification often called for recrystallization from polar solvents or column chromatography to strip away side products—a lesson I learned the hard way after several batches showed stubborn taint from residual reactants.
Dichloro-1,2-thiazole-5-carboxylic acid didn’t just serve as a static component. Its structure saw repeated transformations in my research group, including nucleophilic displacement of chlorine atoms to form substituted thiazole derivatives or coupling reactions taking advantage of the carboxylic acid group for amide or ester formation. The electron-hungry chlorines activate the ring, making it open to attack under both basic and acidic conditions, which unlocks further routes for advanced molecule construction. We also noted that under controlled reduction, selective removal of one chloride could grant access to mono-chlorinated analogues, opening up derivatives with unique biological or catalytic profiles. In modern organic synthesis, the compound’s adaptability paves multiple reaction pathways leading to high-value targets.
Working through catalogs and research articles, this compound often turns up under names such as 3,4-dichloro-5-carboxythiazole or dichlorothiazolecarboxylic acid. Lab supply vendors and chemical distributors sometimes use short names or product codes to streamline cataloging, but any professional ordering or handling this molecule recognizes CAS numbers as the great equalizer for unambiguous identification. Knowing the synonyms helped me quickly cross-reference papers during literature reviews, especially as nomenclature standards sometimes shift between regions and subfields.
Handling dichloro-1,2-thiazole-5-carboxylic acid means taking chemical safety seriously. Direct skin contact or inhalation risks prompt the need for gloves, goggles, and well-ventilated lab environments. Regulatory filings point to acute toxicity, though typical sealed-container transport lowers risk during transit. Our team always made certain that spills got neutralized with basic sorbents and that waste disposal followed established hazardous chemical protocols. Equipment like spill trays, eyewash stations, and chemical-resistant lab coats weren’t suggestions—they shielded colleagues from real accidents. By embedding safety into every step, from the weighing scale to final disposal, the actual risk reduced—a principle at the core of every responsible chemical operation I’ve joined.
Dichloro-1,2-thiazole-5-carboxylic acid captured attention in fields from pesticide synthesis to medicinal chemistry. In agrochemicals, research highlighted this framework as a precursor to fungicidal and herbicidal actives, while in drug development circles, teams probe its ability to introduce functionalization into novel pharmacophores. Even outside the pharmaceutical world, material scientists eye its reactivity for creating electronic and photonic device components. In my research, applying this compound as a starting point led quickly to libraries of new molecular scaffolds with promising anti-infective or crop-protection properties. Academia and industry both value its versatility—when a new catalytic property or biological target emerges, this molecule sits firmly on the roster of ready building blocks.
Labs continue investing in R&D around dichloro-1,2-thiazole-5-carboxylic acid, aiming to extend utility and safety. Screening efforts check for new pharmacological leads while applied scientists stress-test its performance in complex multi-component syntheses. My academic contacts have tested alternative preparation routes, trying greener chemistry methods with less toxic reagents and lower waste. By focusing on scalable and selective processes, research teams seek to provide both cost savings and reduced environmental impact. R&D also chases new reaction partners—broadening the catalog of derivatives that could click with important protein targets or industrial catalysts.
Published reports point to moderate acute toxicity, with both oral and dermal exposure requiring careful limits in workplace settings. While animal studies form the bulk of available data, many companies and universities supplement these findings with in vitro assays to predict broader environmental and human health effects. Affected organs most often include skin, respiratory, and possibly the gastrointestinal tract on direct exposure. In my experience, ensuring airtight containers and fume hood procedures proved essential for protecting both staff and the environment. Modern regulatory regimes demand transparent hazard communication, supporting risk management all the way from research scale to commercial application.
Looking forward, dichloro-1,2-thiazole-5-carboxylic acid holds promise not just for those chasing new crop protection agents or pharmaceutical leads, but also for chemists creating complex frameworks for next-generation devices. Pushes towards sustainable synthesis—particularly use of renewable feedstocks and recyclable process routes—can trim costs and environmental impact at scale. Industry voices predict expanding roles in advanced materials where controlled ring substitution sharpens performance properties. Continued investment in both new applications and safer, greener production pathways could tip the balance, bringing this compound into sharp focus for solving genuine challenges across multiple scientific and industrial landscapes.
Dichloro-1,2-thiazole-5-carboxylic acid doesn’t make headlines. Most people, unless working in a lab or deep into synthetic chemistry, probably never hear its name. Yet, some of the biggest shifts in agriculture and medicine rest on molecules like this. Scientists use this compound mostly as an intermediate in crafting much bigger, often life-changing products.
Over the past decade, global food security has faced tough challenges. Growing enough healthy, resilient crops has become a bigger priority. Here is where dichloro-1,2-thiazole-5-carboxylic acid slips behind the curtain and begins to shape things. Many modern herbicides, which farmers rely on to protect yields from unruly weeds, come from chemical families that use this compound somewhere early in their process. Molecules with the thiazole group help build selective weed killers—chemicals that go after the “bad guys” and leave wheat, corn, rice, and other grains in decent shape.
To put it plainly, without these herbicides, we’d probably see more wasted land, higher food prices, and a much greater fight for every loaf of bread. Experts at the Food and Agriculture Organization estimate persistent weed infestations can slash harvests by up to 34%. By joining in the production of next-gen herbicides, dichloro-1,2-thiazole-5-carboxylic acid plays a quiet but real role in making sure supermarket shelves stay full.
Pharmaceutical research loves complexity, often beginning with simple compounds and adding new pieces, trial by trial, to fight old diseases or treat new ones. Thiazole derivatives crop up all over experimental medicine, especially in developing anti-viral and anti-inflammatory drugs. Dichloro-1,2-thiazole-5-carboxylic acid’s structure brings flexibility; researchers can tweak or attach different groups, searching for a molecule that targets, say, an aggressive infection or a stubborn chronic illness. Some newer generations of antivirals trace their origins to early work with thiazole-based building blocks.
It’s not only about medicine and plants. Polymer chemists and makers of specialty materials also look toward these multi-ring compounds to add heat resistance, boost electrical properties, or create new coatings for electronics. A thiazole group can change how a material handles moisture or stands up in harsh industrial settings.
Nobody can talk about chemicals without thinking about their impact. Manufacturing intermediates like dichloro-1,2-thiazole-5-carboxylic acid asks big questions about waste, toxicity, and long-term safety. Even if this molecule never ends up in the final pill or on the food itself, its footprint goes back to the factory floor.
Every responsible company—especially those aiming to meet modern sustainability guidelines—works on tightening controls, recycling reagents, and limiting dangerous by-products. Strict international laws demand traceability and transparency in how these kinds of chemicals get made and shipped.
Finding safer synthesis routes, cutting solvents, and introducing green chemistry concepts have already shaped how industry approaches molecules like dichloro-1,2-thiazole-5-carboxylic acid. Academic partnerships drive smarter research, so not every solution relies on older, dirtier processes. Greater openness about what’s inside agrochemicals, medicines, and materials keeps trust strong between chemists, regulators, and the wider public.
At the end of the supply chain, most of us will never see or touch dichloro-1,2-thiazole-5-carboxylic acid. But anyone who’s stocked a pantry, taken medicine, or used a modern phone, depends—somewhere along the line—on chemistry that begins with molecules just like this.
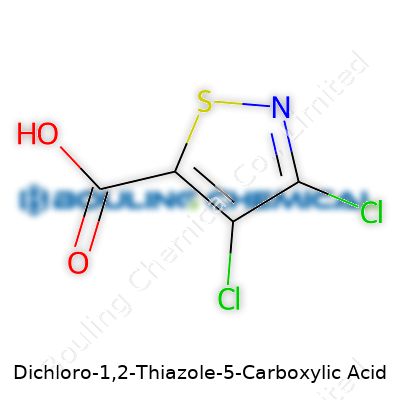
Getting a good look at Dichloro-1,2-Thiazole-5-Carboxylic Acid means picturing a five-membered ring with some heavy chemical substitutions. This isn’t just textbook stuff—it directly shapes physical properties, reactivity, and what chemists can do with it. The thiazole core forms the backbone: a tiny, aromatic ring made from both nitrogen and sulfur. Toss in two chlorine atoms on adjacent carbons, plus a carboxylic acid group nailed to the fifth position, and you've got yourself a molecule that stands out in both academic and industrial circles.
Take a closer look. The thiazole ring starts with one nitrogen, one sulfur, and three carbons. Chlorines cling to the first and second carbons, which makes a big difference for how the whole structure acts. Each halogen tugs on electrons, changing how this compound reacts with other chemicals and even how soluble it is. Sitting at the fifth position, the carboxylic acid group delivers an acidic punch, spinning out implications for biological activity and material processing.
From years spent watching organic chemists at work, real interest in these structures comes down to action, not just appearances. Chlorines bring both power and challenge. Their presence shifts how the thiazole ring can be transformed, especially in drug discovery or crop protection studies. Electron-withdrawing groups like these two chlorines make new derivatives possible by opening up the ring to halogen exchange, coupling reactions, or nucleophilic attacks that simpler thiazoles just won’t allow. The carboxylic acid opens doors for forming salts, amides, or esters, showing up everywhere from chemical synthesis to pharmaceutical design.
Chemists rarely talk about structure without raising the next topic: safety and environmental impact. Halogenated aromatic species, including dichlorothiazoles, often linger in the environment. There’s a trade-off between reactivity and persistence, and that’s not a minor thing. With heavier regulation of halogenated compounds worldwide, labs keep searching for cleaner alternatives or less persistent by-products. Having a robust understanding of chemical structure speeds up predictions about toxicity, persistence, and best disposal practices. Organizations like the ACS or REACH put out strong guidance for these compounds, encouraging greener routes or recycling efforts wherever possible.
The structure of Dichloro-1,2-Thiazole-5-Carboxylic Acid creates doors for both research and new technology, but it doesn’t close off concerns. Chemical innovation grows by looking at design—can chlorines be replaced, or functional groups tweaked for lower toxicity? Researchers take the thiazole core and twist it in new directions: exploring bioactive analogs, more degradable materials, or easier synthesis for scale-up. Building new molecules with tough standards in mind depends on a crystal-clear understanding of that backbone.
Diving into the details brings out the bigger story. It’s not just a chemical curiosity—Dichloro-1,2-Thiazole-5-Carboxylic Acid shapes outcomes in pharmaceutical research, synthetic strategy, and environmental science. Getting a grip on its structure isn’t only for theoretical exercises. It’s what helps real scientists and companies build safer, more effective, and environmentally sound chemicals, right from the first ring drawn on a whiteboard.
Dichloro-1,2-thiazole-5-carboxylic acid isn’t something you want to leave lying around. This compound draws attention for its reactivity and its impact on people who work with specialty chemicals. The storage and handling details make all the difference. Over the last decade, I've worked in academic labs packed with reactive materials, and protocols shape safety just as much as protective gear. You start to appreciate every warning and see why these details exist—sometimes from bitter experience.
This compound acts as a corrosive and sometimes volatile substance. Avoiding moisture and avoiding heat comes straight from its chemical structure: acids, especially those packing halogen atoms, don’t play nice with humidity or high temperatures. Exposure to air with a little too much water vapor can nudge the contents towards decomposition, which pumps out noxious gases. Anyone who's witnessed an acid suddenly "hiss" and fume on opening learns the lesson once.
So, you keep the container sealed tight. Glass or compatible polymer bottles, shielded from sunlight, show up on every best-practices list for a reason. Bright rooms or windows push decomposition along, and nobody wants to discover what gas pressure looks like inside an old, overlooked bottle. I've lost sample vials to this, and cleanup sticks in your mind.
A good chemical storage space features steady, cool temperatures—most experts set their thermostats right under 25°C. Flaming cabinets and explosion-proof fridges exist for good reason, mainly because a few failed experiments have filled many labs with eye-watering smoke. Keep acids far from anything reactive—especially bases, solvents, and metals. One memorable mishap taught my team the cost of letting an acid get too close to an open bottle of amine. The resulting cloud forced a full evacuation.
Labeling forms a strong line of defense. Permanent, chemical-resistant labels tracking date and concentration save hours when you need to audit chemical stock or answer a safety investigation. Regular checks—open those cabinets every month and look for color or odor changes—keep surprises at bay. If a bottle looks off, the right plan is disposal, not risk.
Never underestimate the irritation and burns this acid brings to skin, eyes, and airways. I’ve seen short sleeves and thin gloves end in emergency showers. Heavy-duty nitrile gloves, goggles, and face shields: all non-negotiable. Good fume extraction, preferably a certified hood, guarantees that fumes leave the lab, not your lungs. Every person who spends time on the bench agrees that shortcuts in personal protection create bigger problems.
Despite training, accidents still happen in labs and warehouses. Improving signage, sharing incident reports, and building a culture where people actually speak up about unsafe shortcuts does more than just meet regulations—it genuinely makes daily work safer. Reviewing data about chemical accidents across university and industry settings shows that regular drills and refreshers drop injury rates. I try to hammer home these lessons with every new student or coworker.
Digital inventory systems help—scanning barcodes before every use, tracking lot numbers, expiration dates, and storage history. Technology can enforce discipline where human memory might slip. Working together with auditors, updating safety data sheets often, and holding each other accountable means that safe handling is more than a checkbox; it’s a real practice.
Dichloro-1,2-thiazole-5-carboxylic acid isn’t a staple you’ll spot on a household cleaning label, but it plays an important role across several labs and industries. This compound carries a molecular weight of 209.00 g/mol, which speaks volumes if you regularly work with precise chemical formulations. The CAS number, 117735-65-6, acts like its fingerprint. Scientists punch in that string of numbers when they source this compound online, thumb through inventories, or need airtight identification for regulatory paperwork.
Over the years, mixing up compounds has led to costly mistakes. There’s a lesson every chemist eventually learns: never trust a label alone. Take the case of a mid-sized pharmaceutical lab in 2018 that picked up a batch with a sloppy secondary label, thinking they had the right thiazole derivative. The reaction fizzled, money got wasted, and two weeks slipped by. Mistakes like this are less probable when folks lean on internationally standardized identifiers, like CAS numbers or molecular weights. These numbers keep a business in sync with local and global partners, as well as regulators.
Safety officers often drill this message: know exactly what’s on your shelves. Chemicals bring risks. A single digit off in molecular weight may put dangerous assumptions into play, especially for compounds with close relatives—think of how a misplaced chlorine can change a harmless substance into a hazardous one. Safety data sheets (SDS) use CAS numbers as anchors for hazard info, emergency protocols, and waste management guidelines. Using the correct molecular weight translates to precise calculations for everything from dilution to neutralization. These small details keep people safe and waste streams under control.
Some of the larger research grants and industry partnerships won’t move forward unless documentation checks out. If you’re part of a university team trying to replicate a study, the CAS number ties every material back to verified sources, ensuring others can trust your results. Industries such as agrochemicals and pharmaceuticals demand this rigor. They operate under strict audit trails where a single misstep closes doors to export and patents alike.
Struggling with chemical sourcing mistakes? Invest time in training staff to recognize the power of unique identifiers. Set up digital inventories that use CAS numbers as searchable tags—automation in this area cuts down human error. Build relationships with suppliers known for thorough documentation and quality controls. Transparency in sourcing doesn’t just meet compliance; it supports bigger goals like sustainable innovation and public health.
I've watched new lab staff get frustrated by the seeming bureaucracy of chemical tracking. After spending hours troubleshooting a complex reaction, you realize the value of those small data points. The devil really does hide in the details. Whether you’re scaling up a pilot batch or teaching undergraduates, practicing this attention to detail reinforces a culture of accuracy and trust. Facts—like a compound’s molecular weight and CAS number—may not grab the headlines, but they underpin every good, safe, and ethical decision in the lab.
Molecular Weight: 209.00 g/molCAS Number: 117735-65-6
Dichloro-1,2-Thiazole-5-Carboxylic Acid doesn’t show up in households or classrooms, but it finds its way into research labs and specialty manufacturing pipelines. Many overlook basic safety because the compound sits far from everyday life, but it carries real risks that can affect health fast. You smell something sharp or rotten, like burning hair mixed with acid—don’t ignore it. This compound often brings respiratory irritation in confined spaces. Just a few minutes of exposure can start a coughing fit or even trigger asthmatic episodes for folks with sensitive lungs. Direct contact leaves skin burning and sometimes red and blistered. Eyes sting and water; contact can leave vision blurry or threatened.
Accidents do happen — beakers break, gloves rip, exhaust fans fail — and the results add up. One study on occupational exposures highlighted that thiazole-based compounds raise the chances of dermatitis and chemical burns. Long-term inhalation wears on lung tissue. The trouble grows if you work in places without adequate hoods or where people downplay protective gear. In my own lab days, I watched a colleague brush a small amount onto her glove, shrug it off, and a day later she needed ointment for chemical burns. Just tiny mishaps show how quickly small-scale risks escalate.
Chemically speaking, dichloro substituents bring extra volatility. This means the compound doesn’t play nice with open air; it wants to send vapors out quickly enough to reach noses and airways fast. Sulfur and nitrogen together (in the thiazole ring) mix qualities from both worlds—sulfur irritates, nitrogen increases reactivity. The carboxylic acid group adds a layer of acidity that plays rough with mucous membranes and tissues.
If mixed—accidentally or during a procedure—with strong bases or oxidizers, this material pushes toward violent reactions. Fumes fly, sometimes heat builds enough for pressure to shatter glassware. Gas cylinders, dirty glassware, and even paper towels soaked in solvents can set off small fires or explosions. Too many labs lock this material away and forget to check containers for corrosion or leaks. The 2020 TechSafe Labs review pointed out that about one in ten reported chemical exposures involved improper storage—chemicals kept in the wrong types of plastic or near incompatible substances.
Goggles, gloves, and lab coats are the bare essentials, but for Dichloro-1,2-Thiazole-5-Carboxylic Acid, that’s not enough. I always found that double-gloving (nitrile over latex) cuts the odds of skin penetration when accidents happen. Face shields help during transfers. Fume hoods matter more with this compound than with simple acids—room airflow alone never protects you from unexpected vapor clouds. I saw how fume hoods, checked every six months, stopped three close calls. Know your ventilation.
Don’t skip the basics: label every container and note its purchase and open date. This acid reacts with air and moisture over time, corroding its bottle and causing leaks. Store it away from bases and oxidizers, preferably locked somewhere cool and dry. Keep clean-up kits within reach, and run regular drills on what to do if someone spills or gets splashed. The best labs I worked in treated every transfer and reaction as a potential hazard, planning out steps before they opened the bottle.
Industry continues searching for safer alternatives or less-reactive derivatives, aiming to limit occupational exposure. Companies and labs that invest in regular safety training, robust storage, and routine equipment checks see lower injury rates. By approaching each chemical—no matter how niche—as a threat until proven otherwise, we all reduce the risks. Attention to the little failures, the ones that happen when someone’s tired or rushing, matters more than a hundred “Eye Wash” posters. Experience teaches that with Dichloro-1,2-Thiazole-5-Carboxylic Acid, there are no shortcuts worth taking.
| Names | |
| Preferred IUPAC name | 5,7-Dichloro-3H-1,2-thiazole-4-carboxylic acid |
| Other names |
5-Carboxy-1,2-dichloro-1,2-thiazole 5-Carboxy-3,4-dichloro-1,2-thiazole 1,2-Dichloro-5-carboxythiazole |
| Pronunciation | /daɪˌklɔːroʊ waɪˈtuː θaɪˈəzoʊl faɪv kɑːrˈbɒksɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 145193-92-0 |
| 3D model (JSmol) | `3D structure (JSmol) string for Dichloro-1,2-thiazole-5-carboxylic acid:` ``` C1=C(SN=C1Cl)C(=O)O ``` |
| Beilstein Reference | 146208 |
| ChEBI | CHEBI:144325 |
| ChEMBL | CHEMBL502792 |
| ChemSpider | 876294 |
| DrugBank | DB08374 |
| ECHA InfoCard | ECHA InfoCard: 100.041.338 |
| EC Number | 693-643-1 |
| Gmelin Reference | Gm: 798488 |
| KEGG | C18608 |
| MeSH | D004080 |
| PubChem CID | 127676289 |
| RTECS number | ZT1575000 |
| UNII | J00R5D7SBE |
| UN number | 3261 |
| CompTox Dashboard (EPA) | DTXSID10894152 |
| Properties | |
| Chemical formula | C4HCl2NO2S |
| Molar mass | 207.05 g/mol |
| Appearance | White to off-white solid |
| Density | 1.89 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.9 |
| Acidity (pKa) | 1.8 |
| Basicity (pKb) | 5.42 |
| Magnetic susceptibility (χ) | -38.9 × 10^-6 cm^3/mol |
| Refractive index (nD) | 1.631 |
| Dipole moment | 2.73 D |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH⦵298) | -217.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -528.4 kJ/mol |
| Pharmacology | |
| ATC code | D06BB |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation, toxic to aquatic life |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P301+P312, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 2-1-1 |
| Flash point | > 143.2 °C |
| Lethal dose or concentration | LD50 Oral (rat): >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 500 mg/kg |
| NIOSH | Not Listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg |
| Related compounds | |
| Related compounds |
Chlorothiazole 1,2,3-Benzothiadiazole Thiazole Dithiooxamide Thiourea |