Dibenzopyrrole brings out stories from the early era of organic chemistry exploration. Chemists, enjoying the golden age of aromatic hydrocarbons, took to the lab to push past the simplicity of benzene rings and fused structures. They started tinkering with heterocycles, gearing up for bigger ideas and new discoveries. The compound’s roots stretch back to exploratory synthesis in the late nineteenth and early twentieth centuries, a time alive with breakthroughs that shaped libraries of research chemicals and jumpstarted entire branches of medicinal chemistry. You see the impact through decades of published experiments, as researchers tested, re-tested, and ultimately brought dibenzopyrrole from theoretical sketches to bench-top reality.
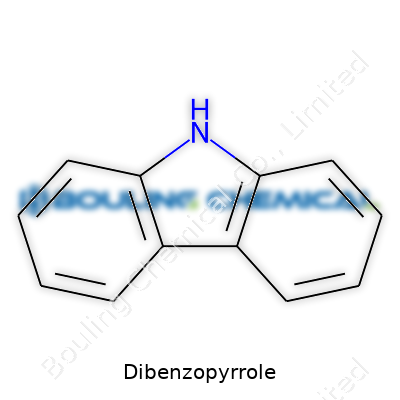
Dibenzopyrrole, also known as carbazole, sets itself apart from common aromatic compounds by merging two benzene rings around a nitrogen atom. This structure forms a tricyclic molecule, giving dibenzopyrrole unique edge in reactivity and light absorption. Today, chemists can pick up bottles of this pale, off-white powder in fine crystalline form. The material heads out to all corners of advanced manufacturing and research, making its way into dyes, pharmaceutical intermediates, and organic electronics. While the public might lump it into "chemical stuff" territory, those familiar with its quirks recognize its role as a backbone for everything from photoconductors to the backbone of some anti-cancer drug molecules.
You tend to notice dibenzopyrrole's excellent thermal stability and resistance to oxidation at room temperature. A melting point just above 245°C catches attention, because it survives varied industrial processes that chew up lesser materials. The compound dissolves in hot organic solvents—think chloroform, benzene, and toluene—but resists water like most of its aromatic cousins. Its nitrogen atom, while tucked away, brings interesting electron-rich character, influencing chemical reactivity and photophysical traits. For spectroscopists and lab workers, its UV absorption profile stands out, laying the groundwork for a host of practical imaging and detection techniques.
Manufacturers label dibenzopyrrole according to purity, appearance, and residual contaminants. Lab-grade powders typically reach 98% or higher purity, with trace metals or halogenated byproducts flagged in the safety datasheet. The CAS number, 86-74-8, acts as its universal ID, clearing confusion for shipping or stockroom requests. Package labels warn about dust formation and demand proper use of gloves, goggles, and respiratory protection. Shelf lives often stretch for years in dry, sealed containers, making inventory management simple for both industrial and academic users.
Old-school chemists once relied on the Borsche–Drechsel cyclization, heating diphenylamine with oxidants or sulfuric acid to coax the rings into forming. Modern synthesis has branched out into palladium-catalyzed cross-coupling and electrochemical methods, which trim down time and waste. High-throughput labs set up parallel reactors, monitoring reaction outcomes by chromatography and NMR. Purification usually means a dance of recrystallization or column chromatography until the powder reaches specification. Big plants scale things up, handling batch reactors with plenty of inert gas and ventilation—no shortcuts on safety.
Dibenzopyrrole’s chemical backbone breathes life into functional group transformations. Halogenation and nitration proceed cleanly, allowing scientists to install substituents for new properties. The aromatic positions invite Friedel–Crafts reactions, while the exposed nitrogen can be alkylated or acylated. These routes turn the molecule into a launching pad for making photoreactive switches, conductive polymers, or medicinal scaffolds. Researchers use its skeleton to prototype materials with improved solubility or higher electron mobility, chasing the next leap in electronic devices or pharmaceutical action.
Dibenzopyrrole travels under many identities. In chemical catalogs and research papers, carbazole dominates, but some texts cite 9H-carbazole or 9-azafluorene. Industrial suppliers lean on the IUPAC nomenclature, yet local safety data often include synonyms like diphenylenimine. Tracking down literature or MSDS entries demands cross-referencing these names, a hassle for new grad students and a real lesson in the value of maintaining tidy chemical records.
Once you’ve spilled even a little of this powder, you notice its dust floats and lingers. Health protocols call for working in a fume hood, double-gloving, and wearing goggles. Inhalation and skin exposure both carry risks, especially after prolonged contact. Brief environmental or regulatory reviews turn up listings for hazardous waste classification, and fire safety labels point to combustion producing toxic nitrogen oxides. Disposal, both in the lab and scale-up settings, strictly follows local regulations. Well-trained technicians and regulatory compliance officers stay sharp, keeping records and providing refreshers to new staff. Lab managers don’t leave its handling to chance—spill kits stay ready, and air monitoring keeps exposure to a minimum.
Carbazole makes real impact in OLED screens, organic photovoltaics, and blue-light-emitting diodes. Manufacturers use dibenzopyrrole derivatives in pigment and dye production, accounting for the stunning colors in printing inks and specialty coatings. Pharmaceutical developers eye its core structure for anti-tumor, anti-inflammatory, and antimicrobial candidates. Environmental labs tap into its photoconductive properties, designing sensors that pick up movement or pollution levels. Just recently, more startups ran with its potential as a building block for biodegradable plastics, a sign that both established and emerging industries treat this molecule as a versatile partner rather than an arcane specialty chemical.
Investigation into dibenzopyrrole expands year after year. Academic labs design new derivatives for improved charge-carrier mobility in field-effect transistors, hoping to leapfrog existing limitations of silicon and metal oxides. Drug discovery teams in pharma companies configure carbazole-based frameworks, adding fluorine or oxygen to achieve higher potency or better metabolic profiles. University spinouts write patents on solar energy applications, fuelled by continuous improvements in molecular design. Industry partnerships with research institutions become common as both sides share expertise and funding, and the publication pipeline stays full of promising new applications—each angled at real-world challenges in health, energy, or materials science.
Toxicologists keep a watchful eye on dibenzopyrrole and its byproducts. Animal studies show the parent molecule holds relatively low acute toxicity, but some derivatives, especially those formed by combustion in industrial settings, carry mutagenic or carcinogenic profiles. Workers exposed to dusts or vapors for long shifts report skin and mucous membrane irritation, and chronic studies raise flags for potential liver and kidney effects. As a result, environmental monitoring of waste streams from dye plants and electronic-component manufacturers includes regular carbazole checks. Pressure builds for greener manufacturing routes, not just to meet regulations but also to answer public demand for responsible chemical stewardship.
Breakthroughs with dibenzopyrrole move fast, reflecting growing ambitions in electronics, sustainable materials, and molecular medicine. Engineers design polymers for flexible screens and wearables, banking on carbazole’s natural conductive edge. Materials scientists explore greener synthesis, looking to shrink waste and energy requirements. On the pharmaceutical side, ongoing clinical trials for carbazole-based antivirals and antitumor agents stand out, hinting at new therapies just over the horizon. Environmental advocates nudge chemists to revisit and reduce toxic byproducts. My experience working with cross-disciplinary teams has convinced me that molecules like dibenzopyrrole won't fade into textbook obscurity. Instead, they’ll anchor new applications, stretching the definition of what organic chemistry can deliver for both industry professionals and everyday consumers.
Most folks probably haven’t heard the word “dibenzopyrrole” outside of a chemistry classroom. The truth is, these kinds of compounds hide in plain sight, woven into the fabric of things like pharmaceuticals and dyes. Somewhere along the way, scientists noticed the special backbone this molecule carries gives it extra punch in how it interacts with chemicals and living cells.
If you open a cabinet in any hospital pharmacy, you’ll probably find derivatives of dibenzopyrrole nearby. Some antidepressants, anti-inflammatories, and antipsychotic drugs trace their origins to this basic structure. What matters here isn’t just the way these compounds block or boost certain signals in the brain. It’s about how one tiny shift in the chemical shape can mark the difference between relief and harsh side effects.
For example, the indole ring system (a type of dibenzopyrrole) appears in tryptophan, a building block for serotonin. No serotonin, and your mood nosedives. The same ring system works as a springboard for drug chemists who want to fine-tune how a medicine stays in the body or targets specific cells. It’s no secret: The reason doctors depend on some of today’s life-saving treatments comes right back to this chemical ring.
Walk past a wall of paints or fabrics dyed a deep blue or violet, chances rise that some of those bold shades rely on molecules closely related to dibenzopyrrole. Certain pigments need this structure to resist fading in sunlight and stay sharp on your clothes and houses. When companies make new plastics or coatings, they often reach for these compounds because they don’t break down fast and can shrug off rough treatment. Having designed stains that can survive a summer beating or a hundred spins in the wash, I’ve seen firsthand how a lab tweak using dibenzopyrrole building blocks keeps colors looking new far longer than the old standards.
In the rush to squeeze more performance from dyes and medicines, it’s easy to lose track of what happens once dibenzopyrrole-based chemicals leave the lab. Some breakdown products carry risks to soil, water, and animal life. Years ago, I helped run a small water-testing project, and we found minute traces of synthetic dyes where they shouldn’t be—from streams near textile plants to neighborhood ponds. Most folks never noticed, but over time, these “forever chemicals” can pile up.
Pulling the plug on useful compounds isn’t something anyone’s asking for. The real need sits in stronger rules and better waste-handling at factories, plus smart chemistry to design drugs and dyes that fall apart safely after their job is done. Big advances can come from making biodegradable versions or using catalysts that break these compounds back down before they hit the environment. I’ve met teams who dig into green chemistry for this reason: the fix starts with inventors and ends with strict oversight.
It’s clear dibenzopyrrole isn’t some obscure footnote in a chemistry textbook. Its reach into healthcare, industry, and even your closet feels huge. The challenge is squeezing all the good from this clever molecule, while keeping a sharp eye on what comes next—whether in a medicine bottle or a stream on a quiet afternoon.
Dibenzopyrrole, or carbazole if you’re browsing through chemical glossaries, keeps coming back into conversations about dyes, electronics, and agriculture. This compound grabs attention because of its deep color, slight earthy smell, and its unmistakable structure—two benzene rings glued to a five-membered nitrogen-containing ring. For folks working in chemistry labs or industries, carbazole stands out. If you have worked with it, you know it isn’t just another white crystalline powder sitting on a shelf.
The naked eye spots carbazole as a white or faintly beige crystal, but try crushing it between your fingers—it’s dry and chalky. At slightly over 245°C, it starts melting, which warns you about its heat tolerance. Unlike sugary crystals, it’s got a firmness that resists crumbling under minor force.
If you pour water over it, you’ll still see the powder staring back at you—carbazole barely dissolves in water. To dissolve it, you’ll need trickier solvents like benzene, chloroform, or toluene. I’ve sat in the lab frustrated, waiting for it to budge, only to realize it prefers the company of aromatic solvents. The near-invisibility in water also means aquatic cleanup requires more careful thought.
Pure carbazole blares a strong blue-violet color if you shine ultraviolet light on it. This property shows its links to industries fixing on organic electronics, especially organic light-emitting diodes (OLEDs). The reason? It throws off a solid fluorescence. Quality control folks use this glowing character to judge purity or, sometimes, catch cheap imitations.
Carbazole’s backbone stores stability thanks to its aromatic rings. The nitrogen site, though, plays a wildcard role. Add some extra chemical push, and it jumps into reactions—especially at the 3-position next to the nitrogen. Whether synthesizing pigments, drugs, or agricultural protectants, this spot gets attacked or swapped with new groups. In organic chemistry circles, nothing throws off experiments faster than ignoring that prime spot.
During my time running reactions with carbazole, the biggest headaches came from its stubbornness with heat and light. Scatter it around in daylight, and nothing much happens. Try igniting it, and clouds of smoke come swirling out. If you’re working with it, keep it away from flames. Its structure shrugs off mild acids and bases, making it tough to degrade. Tossing it into reactions calls for high temperatures or concentrated acids. This trait also means it lingers in the environment, not breaking down quickly.
Chemists, engineers, and factories like carbazole for turning dull plastics into flashy, long-lasting products. Environmental health advocates worry, for good reason, about its persistence and the way it hangs around in soil and water. I’ve spoken with colleagues handling spill cleanups—they’ve learned harsh lessons on its durability. More sustainable solutions might use recycled carbazole, or new catalysts which break it down faster after use. Research is crawling forward, looking for microbes or enzymes that can tackle it, but those options haven’t scaled yet.
Whether glowing under UV lights, refusing to mix with water, or anchoring the foundation of purple inks, carbazole keeps popping up in routines for both chemists and factory workers. Tackling its stubbornness could bring safer handling and cleanup practices, benefiting communities and landscapes nearby. Until then, its remarkable mix of stability and reactivity will keep shaping both industry products and cleanup conversations.
Most of us learn the dangers of chemicals through grim stories. A broken bottle in a research lab. Someone ignoring the warning label, thinking the rules are made for someone else. Dibenzopyrrole, a compound showing up in dye manufacturing and pharmaceuticals, comes with its own share of real hazards. Organic compounds with these ring structures often escape notice, yet their fumes and dust can hit you harder than you might expect. The best strategy involves facing those hazards directly.
Back in my early chemistry days, we stored a lot of chemicals on open shelves, lids loose, brown bottles crowding for space. We trusted labels and hope—a risky combination. Dibenzopyrrole shouldn’t live that way. Closed containers matter. Glass with tight-fitting caps trumps plastic when there’s any risk of leaching or vapor loss. Thick, opaque bottles protect this moisture-sensitive compound from light and air. I’ve seen what a little humidity does to materials like this: cake the powder, mess with purity, and sometimes spark dangerous reactions.
A cool, dry, ventilated space beats the back of a drawer any day. Avoid the chemical soup effect—separate this stuff from oxidizers and acids. A single misstep could ruin years of work or, worse, send someone to the hospital. Temperature swings mess up stability. Room temperature works, but closets near heat vents don’t. I always recommend using labelled, locked cabinets away from common traffic. Someone will always cut corners for convenience. Don’t leave temptation out on the bench.
Textbooks say “wear gloves, goggles, and a coat.” Real safety means putting on that gear every single time, not just for inspections. Dust settles on your desk and on your skin. I watched too many colleagues shrug about fumes and skip the hood, only to fight headaches or worse the next day. Fume hoods aren’t just for show. They suck up those invisible threats before lungs or skin take the hit.
Sometimes nitrile gloves do fine, but check the manufacturer’s chart—some glove materials only buy you a few minutes’ safety. I keep extra pairs nearby, just in case. Lab coats need washing. The dust sticks around, and dragging it home serves no one.
Nobody brags about proper waste disposal, but skipping it builds up problems fast. I’ve seen too many sink drains and trash cans become chemical time bombs. Dibenzopyrrole isn’t something to wash out or throw out with yesterday’s coffee cup. Seal the waste in compatible containers, label it clearly, and use a collection service.
Spills happen, and how you react makes all the difference. Good practice sets up spill kits, absorbent pads, and written steps nearby. Don’t fumble through an emergency. Take time to train everyone, even visitors. Knowing what to do means fewer bad outcomes.
The checklist approach—posters, reminders, digital logs—helps cut down on mistakes. Ventilation upgrades, regular inspections, and accessible personal protective equipment turn best practices into daily habits. I learned the hard way that old habits can lead to emergencies, but nothing beats a workplace where everyone calls out unsafe shortcuts.
Dibenzopyrrole doesn’t forgive carelessness. Consistent training and honest conversations about risks turn corners into safe zones. Own your habits, and encourage others to do the same. That’s how you keep yourself and your coworkers safe, every shift.
Anyone who works with chemicals knows the routine: wear gloves, goggles, work under the hood, don’t take shortcuts. I remember hauling cases of solvents during a summer in a small research lab, always anxious about what the older, obscure substances could do. Dibenzopyrrole, better known among chemists as carbazole, tends to show up on hazard data sheets. Breathing it in, or letting it get on skin, can lead to irritation. Lab tests also remind us that dibenzopyrrole’s breakdown products, especially if you burn it, don’t lead to anything benign.
A few studies point at risks that go further. Lab rats exposed to high doses saw some changes in their organs over time. It triggers concern whether this substance could spark cancer or disrupt hormones. So far, most cases involve workers or researchers, not ordinary folks, which makes oversight in industries essential. Still, it pays to keep an eye on substances that stay under the radar, especially if regulations lag behind new findings.
Once dibenzopyrrole finds its way into soil or water, it likes to stay put. It resists breaking down, which means it lingers around much longer than a lot of simple compounds. I’ve seen that persistence turn into trouble along riverbeds where chemical plants once dumped their sludge. Wildlife doesn’t care about the source; it just suffers the effects. Some types of fish and plant life react to accumulated toxins by disappearing or failing to breed properly.
Even though dibenzopyrrole isn’t the worst offender, it contributes to a toxic load when combined with other chemicals. Picture a stew of residues building up year after year. Municipal filters struggle to remove these kinds of molecules, so traces can show up far from the site of original contamination.
Many dyes, pharmaceuticals, and plastics have roots in the same chemistry as dibenzopyrrole. That makes oversight—real, boots-on-the-ground monitoring—critical. Too often, outdated safety standards let chemicals drift into communities. My own town fought for years to get a nearby plant to share real chemical inventory records. Without that, it’s tough to know what’s in the air and water.
Kids and elderly folks deal with exposure in ways healthy adults don’t. Chronic exposure at low levels—what comes with living near factories—boils down to a numbers game filled with risks that grow over time, not overnight.
Sweeping reform rarely fixes these problems. Change builds from bottom-up steps. Environmental groups can push for regular monitoring, forcing companies to test local rivers and soil, and share that data with residents. Companies could lower emissions through better filtration, or switch out outdated equipment that leaks or exhausts harmful vapors.
Individual workers get it: better ventilation, modern protective gear, clear instructions save lives. Researchers looking for greener chemistry can design substitutes that don’t stick around in the environment. Community pressure can tilt company policy when enough people demand accountable storage, disposal, and cleanup.
Dibenzopyrrole won’t vanish from industrial use overnight. Honest reporting, stronger controls, and a dose of public stubbornness can keep it from sliding into that long list of “forgotten hazards” that only haunt us after the damage shows up.
Dibenzopyrrole stands out among polycyclic molecules for its versatility in both research and pharmaceutical contexts. Chemists who create this compound in the lab stick to classic protocols, often following the Fisher indole synthesis or Buchwald–Hartwig amination. It’s easy to look up named reactions, but actually pulling off a clean synthesis in a flask comes with headaches. Quality glassware, patient refluxing, and meticulous attention to temperature make or break the outcome. Using indole-based precursors, people mix reactants under an inert atmosphere, avoid moisture, and time the heating stage well. The top labs keep their solvents ultra-dry, steering clear of water that ruins yields. This hands-on, practical side matters more than any abstract recipe list.
Not everyone works in a modern lab or has funding to burn. Getting your hands on rare starting materials, like diphenylamine or specific halides, can stall the whole process. Picture chasing the right reagents across catalogs or navigating import rules that treat legitimate scientists like troublemakers. Chemical suppliers do carry dibenzopyrrole, but expect paperwork and background checks that drag on. Prices swing, and, in my own experience, small quantities often come with steep markups. If you’re running a tight ship or stuck at a university with limited grants, this can put your projects on ice for weeks, even months.
Some think just getting dibenzopyrrole is enough, but sloppy synthesis leads to contaminated product. I once ran a reaction without a fresh desiccant – the yield tanked, and so did the purity. TLC plates, melting point analysis, and NMR checks save wasted effort downstream. There’s little point in running advanced bioassays on an impure sample pretending to be dibenzopyrrole. Anyone who spent hours extracting product from a dirty reaction knows this lesson the hard way. Good labs double up on verification, and smart chemists never rely on the vendor’s word alone.
To cut down risk and hassle, some teams join consortia to pool resources, split reagent costs, and share expertise. I’ve seen university departments thrive this way, making the most of every budget line. Outsourcing synthesis to a reliable contractor brings another route, especially for groups without the right infrastructure. Working with trusted CROs keeps the process efficient and transparent, though it brings extra cost and less hands-on control. For those facing strict oversight or import limitations, establishing clear relationships with certified suppliers helps keep shipments legal and above board.
Demand for dibenzopyrrole looks set to rise in multiple sectors. By building local supplier networks and advocating for streamlined chemical regulations without sacrificing safety, scientists and industry can both benefit. Investing in training for younger chemists on solid lab technique pays off in fewer failed batches, more robust results, and less wasted time. In my own work, swapping tips in seminars and sharing leftover chemicals within the lab community helped bridge gaps between opportunity and access. Real collaboration beats every shortcut.
| Names | |
| Preferred IUPAC name | Carbazole |
| Other names |
Carbazole 9H-Carbazole |
| Pronunciation | /daɪ.bɛn.zəˈpaɪ.roʊl/ |
| Identifiers | |
| CAS Number | 85-26-9 |
| Beilstein Reference | 120873 |
| ChEBI | CHEBI:35581 |
| ChEMBL | CHEMBL998 |
| ChemSpider | 55045 |
| DrugBank | DB04744 |
| ECHA InfoCard | 100.016.137 |
| EC Number | 206-116-6 |
| Gmelin Reference | 84778 |
| KEGG | C06535 |
| MeSH | Dibenzopyrroles |
| PubChem CID | 6814 |
| RTECS number | DU8050000 |
| UNII | QX28P2QABJ |
| UN number | UN2209 |
| CompTox Dashboard (EPA) | DTXSID4062215 |
| Properties | |
| Chemical formula | C12H9N |
| Molar mass | 219.27 g/mol |
| Appearance | White to light yellow crystals |
| Odor | Odorless |
| Density | 1.18 g/cm3 |
| Solubility in water | Insoluble |
| log P | 2.68 |
| Vapor pressure | 0.000101 hPa (25 °C) |
| Acidity (pKa) | 23.0 |
| Basicity (pKb) | 11.56 |
| Magnetic susceptibility (χ) | -68.0e-6 cm³/mol |
| Refractive index (nD) | 1.682 |
| Viscosity | Viscosity: 2.46 mPa·s (25 °C) |
| Dipole moment | 3.48 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 243.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | +151 kJ mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -6172.0 kJ/mol |
| Pharmacology | |
| ATC code | N05AX08 |
| Hazards | |
| Main hazards | May cause respiratory irritation. May cause skin irritation. May cause eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| Flash point | 113°C |
| Autoignition temperature | 650°C |
| Lethal dose or concentration | LD50 oral rat 530 mg/kg |
| LD50 (median dose) | LD50 (median dose): 220 mg/kg (oral, mouse) |
| NIOSH | RA3850000 |
| PEL (Permissible) | 1 mg/m3 |
| REL (Recommended) | 1 mg/mL in methanol |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Carbazole Indole Acridine Phenoxazine Phenothiazine |