Back in the mid-20th century, a scramble existed to develop safer and more efficient organic peroxide compounds for industrial reactions. Chemists looking to tune the performance of radical initiators landed on di-tert-pentyl peroxide. They saw plenty of trial and error, given the stubborn reactivity and tricky handling of peroxides in general. Commercial synthesis didn’t take off until improvements in purification and the stabilization of tertiary alkyl peroxides unfolded, something that came arm-in-arm with a clearer understanding of free radical chemistry and tight standards for storage and shipping. With the chemical industry hunting for alternatives to di-tert-butyl peroxide—especially in polymerization and fine chemical synthesis—di-tert-pentyl peroxide entered manufacturing catalogues in the 1960s and achieved a rather niche but persistent foothold.
Di-tert-pentyl peroxide shows up as a pale liquid. Its use comes down to consistent radical generation, making it popular in applications calling for that specific functionality: from polymerization all the way to stepping up certain oxidation reactions. It performs well under elevated temperatures, earning respect among chemists and engineers who want reliable free radical sources that don’t decompose too fast or too soon. Makers package it in tough containers with proper venting, aware that mishandling can spell disaster. Still, its relatively lower volatility compared to similar peroxides nudges it ahead for specialized jobs.
On the chemical front, di-tert-pentyl peroxide has the formula C10H22O2 and a molecular weight a bit north of 174 g/mol. It sports a boiling point typically around 150°C (at standard pressure), though handling almost never happens near that temperature. Flash point creeps up to about 30°C, underlining the need for careful temperature regulation. Density sits close to 0.78 g/cm³, and its solubility drops quickly in water, but mixes well with many organic solvents. Above all, its bond dissociation energy—responsible for radical formation—is high enough to keep it shelf-stable under cool, dark conditions, but low enough that it springs into reactivity when pushed with a bit of heat or a catalyst.
Any barrel or flask marked “Di-tert-pentyl peroxide” runs with strict hazard designations under the GHS system. You’ll spot orange or red diamond pictograms, and a UN number that spells its identity at customs and in shipping manifests. Transport rules treat it like fire in a bottle, mandating insulated, climate-controlled freight and clear placards on trucks or rail cars. Most suppliers guarantee product purity above 97%, with stabilization by small amounts of added slow-reacting hydrocarbon. Paperwork always comes with detailed guidance on shelf-life, decomposition temperatures, and spill management—no room for mistakes.
Chemists prepare di-tert-pentyl peroxide by a straightforward alkylation strategy. They combine tert-pentyl alcohol with hydrogen peroxide, then tweak reaction conditions with acid catalysts—often sulfuric acid—to nudge the peroxide bond formation. After extraction, careful vacuum distillation follows, a step that calls for hands-on experience and a nose for risk, since runaway reactions remain a constant threat. Each batch gets tested for leftover acid, unreacted alcohol, and thermal stability before making it to the shelf. Attempts to improve yields focus on more efficient catalysts and in-line monitoring for heat spikes, since most accidents flow directly from undetected hot spots.
Di-tert-pentyl peroxide stands as a workhorse initiator in free radical chemistry. It cracks at around 140°C–180°C, producing two tert-pentoxy radicals. Those fragments spark a series of chain reactions in polymers or drive oxidations in organic synthesis. Unlike its close cousin di-tert-butyl peroxide, this compound offers a slightly different balance between stability and reactivity, allowing for finer control in some industrial chains. Researchers also tinker with blends, substituting tert-pentyl for other alkyl groups and observing shifts in reactivity. Even though its modifications don’t always guarantee safer handling, they open up new doors in specialty rubber and plastics.
In the catalogues and customs paperwork, di-tert-pentyl peroxide hides behind several aliases. Names like bis(1,1-dimethylbutyl) peroxide, DTPOP, and 2,2,5,5-tetramethyl-3,4-hexanedione peroxide often pop up. These synonyms cover the same molecule, but labeling shifts with supplier and region. It’s crucial to double-check product codes, as mixing up one peroxide for another can make a world of difference in both performance and risk.
Handling di-tert-pentyl peroxide borders on a ritual. Synthetic chemists, plant operators, and logistics crews go through rounds of training. Facilities keep temperature logs, and containers store in specialized bunkers separate from acids, bases, and reducing agents. Fire suppression systems lean heavily on non-water varieties to keep peroxide decomposition from spreading. Engineering controls try to minimize vapor formation. Spills or leaks mean evacuation and immediate clean-up, always by personnel in full protective gear. The Chemical Safety Board and OSHA spell out strict standards to prevent catastrophic events, and recent revisions ask for real-time monitoring systems in any facility storing bulk quantities. Regular drills and up-to-date safety data sheets remain front-line tools against human error.
Biggest users of di-tert-pentyl peroxide line up in plastics. PVC producers and specialty rubber processors lean on its radical-forming power. Cable sheathing, automotive parts, drainage pipes—any application demanding strong polymer backbones sees this peroxide working behind the curtain. The fine chemicals industry calls on it for selective oxidations or ring opening reactions, especially where gentle, controlled radical activity beats harsher oxidants. Small-scale labs deploy it for making custom monomers or exploring tough-to-crack carbon bonds. Its place in the world may look small, but swapping it out can cost efficiency, product quality, and sometimes even safety.
Teams keep hunting for safer versions that pack the same punch. Green chemistry advocates test catalysts that reduce peroxide waste and lower overall hazard, but so far, traditional preparation routes dominate. Cross-industry collaborations hope to develop stabilizing additives, smart packaging that signals decomposition, and digital monitoring for temperature excursions in real time. Custom blends with altered alkyl groups might bring new performance, aiming for both better safety and greater selectivity. Academic labs pursue the fine details—mapping every radical pathway, tracking impurities, and modeling thermal degradation—to spot issues before they spiral into product recalls or, worse, factory incidents.
Toxicologists study di-tert-pentyl peroxide with a wary eye. While direct exposure risks stay lower than some more volatile peroxides, breathing in its vapors or splashing liquid leads to irritation, burns, and sometimes longer-term respiratory problems. Animal studies pin down a moderate acute toxicity, with long-term impacts less thoroughly mapped, mostly because so much of the compound gets handled in closed reactors. The most acute risk has always been fire or explosion. Modern standards require real-time leak detection and regular medical checks for those in constant contact. Longstanding worker complaints about headaches and skin discomfort nudge companies to keep limits low and invest in better ventilation.
The future for di-tert-pentyl peroxide depends on how well new technology can shoulder its mix of risk and reward. Polymer chemists imagine automated micro-reactors, where dangerous peroxides stay sealed inside small systems, run remotely and with next-level sensors. Environmental regulators push for alternatives with smaller hazard profiles or, in emerging economies, for better education and control over existing hazards. If researchers can prove out more environmentally friendly initiators or figure out waste capture methods that actually scale, the market might evolve. Still, for now, its track record of known performance and reliability means it likely stays in the production toolkit—at least until something genuinely safer and just as effective comes along.
Most people have never heard of di-tert-pentyl peroxide unless they work in a lab or a plant. The name alone makes it sound like something to keep locked in a vault. Yet this chemical shows up behind the scenes in several manufacturing sectors. Most of what I’ve learned comes from working near chemical plants and from talking to folks who understand how certain chemicals drive the nuts and bolts of modern life.
Di-tert-pentyl peroxide stands out for its role in making polymers. If you’ve ever used a plastic bottle or a foam cooler, you’ve handled something influenced by this type of chemical. Manufacturers add it during the process that turns small molecules, called monomers, into long plastic chains. This is called polymerization. The chemical does its work by breaking apart at a fairly low temperature and releasing energy, which helps these chains snap together.
Factories rely on it to kick-start the production of plastics like polyethylene and polypropylene. These aren’t rare products — they show up in packaging, pipes, and automotive parts. The result: tougher and more consistent materials that hold up under repeated stress. Without these kinds of chemicals, making such robust plastics at a large scale would get a lot trickier, and the products themselves wouldn’t be as dependable.
Tires, gaskets, and some sporting goods owe their flexibility and bounce to rubber blending. Di-tert-pentyl peroxide helps rubber makers crosslink polymer chains. In other words, it creates bridges between the rubber strands. The end product resists heat, cracking, and wear. Compared with older methods, this process cuts down on unwanted side products, which keeps everything cleaner and more controlled.
I once met a tire plant supervisor who swore by modern peroxides like this one. The switch helped them meet stricter safety and durability standards without hiking up costs. That kind of improvement matters when those tires carry families or haul freight across highways every day.
Some industries use chemicals like di-tert-pentyl peroxide as catalysts. In chemical manufacturing, reaching high temperatures and using harsh acids puts pressure on equipment and people alike. This peroxide helps trigger reactions at lower temperatures. Less energy burned means lower bills and sometimes even fewer emissions. Companies value any route that cuts waste while keeping quality in check.
Anyone who works with di-tert-pentyl peroxide gets training about its dangers. It’s not something to handle lightly, as it can react in unpredictable ways if left alone in warm storage or around the wrong materials. Factory workers need clear labeling, tight process controls, and strong safety routines. Groups like the Occupational Safety and Health Administration (OSHA) publish best practices for handling and storing such materials. No one wants a chemical accident on their watch.
Safer alternatives sometimes exist, but few match the combination of performance and cost. The challenge rests in balancing chemical innovation against worker and environmental safety. Industry players who invest in ongoing training and automation tend to see fewer mishaps and better product results in the long term.
The pressure grows each year to produce stronger materials and use less energy. Peroxides like di-tert-pentyl peroxide help keep manufacturing moving in that direction. Whether such chemicals stay relevant will depend on finding ways to keep them both effective and safe, for everyone who relies on modern materials.
Di-Tert-Pentyl Peroxide doesn’t show up in a beginner’s kit. This chemical carries some serious risks mainly because it’s a strong organic peroxide. Decades ago, I watched a chemist underestimate a much weaker peroxide and an accidental spill triggered a small fire. Those moments teach people to respect this compound before getting any closer. Knowing these dangers pushes safety from theory to necessity.
Anyone handling this peroxide needs real personal protection. I remember those clear goggles fogging under pressure when working with oxidizers, but eyes come first. Choose splash-proof goggles, a splash-resistant lab coat, nitrile gloves, and closed leather shoes. It’s easy to forget the lab coat’s sleeves before the first pinhole breach—then the skin burn and fear hit hard. There are no shortcuts with gloves either, since this substance can pass through some materials. Nitrile gloves checked for holes each time offer a reliable line of defense. Heavy gloves or double-gloving makes sense during more dangerous tasks like transferring.
Di-Tert-Pentyl Peroxide wants a cool space, away from sunlight, open flames, or other flammable chemicals. Years working near stored peroxides left a habit—a glance at the thermometer, then the labeling. Fires from organic peroxides jump fast. Locked cabinets, vented to prevent gas buildup, should sit far from acids or incompatible reactives. Reliable labels and inventory checks aren’t just paperwork—they avoid confusion. Shelf life matters. If containers show crystal buildup or discoloration, disposal is the only safe move. It’s not paranoia—it’s hard-earned routine.
Ventilation decides whether a routine task stays safe. I once tried prepping a tiny batch in a crowded stockroom, thinking, “It’s just a minute.” The sharp scent kicked in, coughing started, and lessons followed. Always opt for a certified fume hood, never work open on a bench. Even a small spill or rapid evaporation can overwhelm the air and turn toxic. If a larger release occurs, don’t hesitate—leave the space and call for emergency help.
Mixing Di-Tert-Pentyl Peroxide with other chemicals becomes risky fast. Static sparks, friction, or heat can set things off. I learned early to ground glassware, skip metal spatulas, and avoid squeezing bottles. Stir gently, keep temperatures below recommended thresholds, and never rush. Always use plastic or borosilicate glass utensils. If spills happen, use spill kits specifically designed for peroxides—never organic matter or sawdust, which might make matters worse.
No sense working with this chemical unless an eye-wash station, emergency shower, and fire extinguisher are within sight. The extinguisher must suit chemical fires. The neighbor across the bench once froze in shock during an incident, so regular drills keep everyone sharp. Spills, fires, or chemical burns don’t wait for a textbook response. Emergency numbers should hang right by the work area. Waste goes in approved containers, isolated until safe removal. Years in this field make you double-check everything—once after the shift, and again before walking in tomorrow.
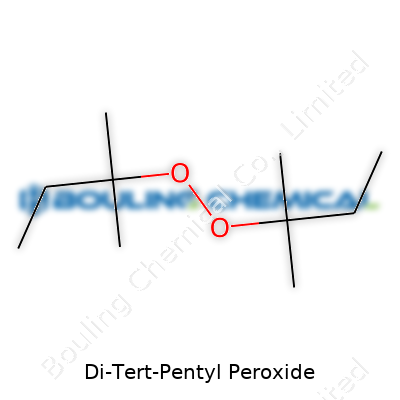
Di-tert-pentyl peroxide stands out in the world of organic chemistry as a dialkyl peroxide. Each side of the oxygen bridge holds a bulky tert-pentyl group, making this compound interesting both in terms of stability and reactivity. Its formula doesn't take long to learn: C10H22O2. Every piece of that formula relies on the arrangement of carbon, hydrogen, and oxygen.
Structure tells its own story. Each tert-pentyl group connects through a central carbon that's bonded to three other carbons and a hydrogen, forming a branched arrangement: (CH3)3CCH2-. The peroxide bridge linked to them is an -O-O- group, soft-spoken, yet explosive under the right conditions. The IUPAC name for this material is 2,4,4-Trimethyl-2-pentyl peroxide, which might pop up in technical documents.
On paper, the structure looks like this: (C5H11)2O2. Imagine a central oxygen-oxygen bond holding two identical tert-pentyl arms. Each arm has a highly branched chain, packed with carbon and methyl groups, making the molecule bulky. That size has practical impact. Big, branched peroxides like this don’t decompose as easily as leaner, more linear molecules.
Handling this chemical takes some respect. Dialkyl peroxides hold potential energy in their oxygen-oxygen bond. If bumped or heated, that bond can split apart, each piece taking an electron and creating free radicals. These radicals can touch off powerful reactions—in industry, that energy finds use in polymerization or as an initiator. For decades, the knowledge of these shifts in structure and energy has shaped safe storage and use, especially in places where temperature control isn’t always guaranteed.
It’s more than just dots and lines. This peroxide’s branched bulk protects it from breaking down too quickly, especially compared with slim, linear peroxides. That doesn’t mean it loses all risk. Any peroxide, with that O–O bond, remains a potential source of rapid energy release. The structure can make storage somewhat safer, but human factors—poor labeling, rough handling, inattentive storage—undo those protections.
Industry guidelines from OSHA and the National Fire Protection Association reflect all of this. Their warnings aren’t theoretical. In the 1980s, a plant experienced a fire traced to improper temperature control of dialkyl peroxides. Learning from those mistakes changed how chemical workers see peroxide storage, inspection, and transport. Reliable chemical suppliers highlight that di-tert-pentyl peroxide’s stability at room temperature should never excuse complacency. Employees receive training to recognize the hazards, and companies invest in explosion-proof storage areas.
Staying safe starts with clear protocols. Labeling stands as a first defense. Training workers to recognize the peroxide’s risks and teach emergency responses provides a second. Regular checks on storage temperature and humidity go a long way to preventing issues. Some labs install real-time sensors, giving everyone another level of reassurance. Technology offers backup, but frontline awareness is what stops most incidents.
Reactivity sometimes draws interest for making new materials. Laboratories experimenting with di-tert-pentyl peroxide usually do it behind blast shields and under controlled ventilation. Chemists resort to personal experience, not just textbooks—hands-on safety drills, reviewing updated literature, and peer consultation help keep best practices fresh. Structure and formula may appear in textbooks, but responsible handling comes from attitudes built over time, both inside and outside the lab.
Di-tert-pentyl peroxide doesn’t show up often in the average household, but it’s a regular player in certain industries, like plastics and rubber. Over the years, anyone who’s spent time near labs or warehouses remembers stories about organic peroxides gone wrong—small leaks filling a room with sharp smells, or worse, surprises with sudden heat. One lesson always stands out—this is a chemical that rewards careful handling and good habits.
Heat makes di-tert-pentyl peroxide dangerous. It breaks down faster as temperatures climb, with potential to start fires or explosions if left unchecked. Direct sunlight increases this risk, pushing the chemical beyond its comfort zone. For that reason, cool and dark rooms become more than just a guideline. Trust in cooling equipment, and check those thermometers often. If air conditioning ever fails, move fast—never leave the container sitting in a hot warehouse hoping it’ll be fine by morning.
Air carries moisture and stray sparks. All it takes is an unnoticed drip, and soon you have decomposition and increased pressure inside the container. That’s why containers should be sealed tightly each time they're opened. Never repurpose a container, even if it looks clean. The original packaging gets tested for resistance, so those standards matter.
Anyone with hands-on experience in chemical storage remembers sorting shelves by compatibility. Di-tert-pentyl peroxide doesn’t get along with strong acids, bases, or reducing agents. Mixing or even storing near these chemicals brings more danger. Try using locked, clearly labeled cabinets, and never let smaller bottles drift across storage zones. Organize areas with simple signage—diagrams help workers sort at a glance.
Sprinklers and extinguishers rated for chemical fires deserve a place close to the storage area. Foam blankets work better than water for peroxide-based fires. Emergency showers and eye-wash stations support anyone handling the chemical, no matter how careful they try to be. Keep exits open and block free from clutter. Local fire departments should know about the stock on hand, so if trouble comes, help arrives faster.
Every chemical hazard control plan counts on people. In practice, training sticks best with stories and clear visuals. If a new worker joins, let them handle the substances under supervision. Practice spill drills as a team, not as a checkbox exercise. Record-keeping tracks quantities in storage, so inventory checks stay simple and up to date.
Safety data sheets shouldn’t live in a drawer. Put them on display, print out the parts about first aid and storage for easy reference, and keep extra copies in the break room. Regulations change, so updates from suppliers become part of the routine—never treat them as junk mail.
Safer storage comes down to commitment, not gadgets. Automate alarms for temperature and leaks in bigger facilities. Regular audits keep everyone aware of the current state of things and push for solutions before cracks show up. Investing in good habits saves property and lives—anyone who's worked around chemicals carries proof of that in their stories and scars.
Di-Tert-Pentyl Peroxide makes chemists and safety officers pause, and for good reason. This organic peroxide acts as a powerful initiator in polymerization, but it doesn’t come quietly. It’s volatile, flammable, and can decompose with heat, friction, or contamination. Anyone who’s opened a drum knows the faint, sharp odor that signals strong chemical activity. A small spark, open flame, or even packing the substance too tightly can trigger an explosion that leaves more than just a scorch mark—a real threat in labs or factories.
Skin contact often brings stinging and redness, sometimes blisters. The liquid can soak through clothes, causing a chemical burn that demands immediate attention. Eyes never stand a chance—splashing can mean permanent damage if not flushed out quickly. Breathing in the vapors doesn’t just irritate your nose; it can hit your lungs and lead to coughing, headaches, or even dizziness in a poorly vented space.
Organic peroxides like Di-Tert-Pentyl Peroxide deal out risk in two main ways: fire and personal exposure. Several industrial fires have stemmed from poor storage conditions. Temperature swings and sunlight give this chemical energy, enough to kick off decomposition. In 2017, the Arkema plant in Texas delivered a hard lesson—when refrigeration failed, organic peroxides decomposed, igniting a toxic blaze. That wasn’t this exact peroxide, but the risk pattern is the same.
OSHA and NIOSH data agree that routine chemical handling sends thousands to emergency rooms each year. Loss of sensation, deep skin burns, and respiratory distress are not rare outcomes when safety takes a back seat. Protective gear, trained staff, and thoughtful procedures aren’t just checkboxes—they save limbs, lungs, and lives.
The best approach starts with treating every drop and vapor as an emergency. If Di-Tert-Pentyl Peroxide lands on skin, strip contaminated clothing and run cool water hard over the area for at least fifteen minutes. Soap helps, but rinsing matters more. Cover the spot with clean gauze after drying, but leave blisters intact to avoid infection.
Splash to the eyes means speed counts. Open the eyelids and flush with water, using an eyewash station or even a sink, for at least fifteen minutes. Turning the head helps drain the water away from the other eye. Contacts need removal—otherwise, they trap the chemical against sensitive tissue.
If someone breathes in a heavy dose, they need fresh air right away. Any sign of drowsiness, coughing, or shortness of breath calls for medical help. Give oxygen if available and trained to provide it. Don’t let the person walk off on their own—monitor until a professional checks them over. Swallowing this chemical demands poison control and quick transport to a hospital. Inducing vomiting at home risks burns to the throat and mouth.
No substitute exists for strong safety culture. Store Di-Tert-Pentyl Peroxide in cool, fire-resistant spaces, away from other chemicals that spark reactions. Keep fire extinguishers suited for chemical fires within reach, and train staff—dry runs save confusion in a real crisis. Safety goggles, gloves, and flame-retardant coats act as shields, not suggestions. Ventilation clears out dangerous fumes before they reach noses or lungs.
New staff deserve hands-on training, not just binders. Drills, labels, and buddy systems catch mistakes before they become emergencies. It’s not about fear—just respect for a substance that rewards caution with a job well done and everyone heading home safe at the end of the shift.
| Names | |
| Preferred IUPAC name | 3,6-Dimethyl-3,6-di(tert-pentyl)-1,2,4,5-tetroxane |
| Other names |
DTPO Bis(1,1-dimethylpropyl) peroxide Bis(tert-pentyl) peroxide |
| Pronunciation | /daɪ-tɜːrt-ˈpɛntɪl pəˈrɒksaɪd/ |
| Identifiers | |
| CAS Number | 630-19-3 |
| Beilstein Reference | 1718731 |
| ChEBI | CHEBI:87173 |
| ChEMBL | CHEMBL138737 |
| ChemSpider | 12606 |
| DrugBank | DB14049 |
| ECHA InfoCard | 03b295af-6d15-4c04-a1a4-7aebf70cfd27 |
| EC Number | 208-704-1 |
| Gmelin Reference | 7548 |
| KEGG | C19684 |
| MeSH | D004222 |
| PubChem CID | 11240 |
| RTECS number | RG2240000 |
| UNII | L859D3D9RN |
| UN number | 3103 |
| CompTox Dashboard (EPA) | DTXSID3022642 |
| Properties | |
| Chemical formula | C10H22O2 |
| Molar mass | 146.26 g/mol |
| Appearance | Colorless liquid |
| Odor | odorless |
| Density | 0.792 g/cm³ |
| Solubility in water | Insoluble |
| log P | 3.8 |
| Vapor pressure | 0.91 mmHg (20 °C) |
| Magnetic susceptibility (χ) | -8.03 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.398 |
| Viscosity | 2 mPa·s (25 °C) |
| Dipole moment | 2.08 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 253.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -398.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -8903.8 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07, GHS09 |
| Pictograms | GHS02,GHS07 |
| Signal word | Danger |
| Hazard statements | H242, H302, H312, H332, H400 |
| Precautionary statements | P210, P220, P221, P234, P280, P305+P351+P338, P310, P370+P378, P403+P235, P410, P411+P235, P420, P501 |
| NFPA 704 (fire diamond) | 3-4-2-OX |
| Flash point | below -20 °C |
| Autoignition temperature | 210 °C (410 °F; 483 K) |
| Explosive limits | Lower: 1.1%, Upper: 7% |
| Lethal dose or concentration | LD50 oral rat 6000 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Di-Tert-Pentyl Peroxide: "400 mg/kg (rat, oral) |
| PEL (Permissible) | '1.5 ppm (10 mg/m3) as TWA' |
| REL (Recommended) | Liquid organic peroxide, Type F, temperature controlled |
| IDLH (Immediate danger) | 150 ppm |
| Related compounds | |
| Related compounds |
tert-Amyl hydroperoxide Di-tert-butyl peroxide Methyl ethyl ketone peroxide |