Look back a few decades, and lab benches didn’t have Di(Succinimido) Carbonate nearly as often as you see it today. This compound has roots in the late 20th century as researchers sought safer, more selective agents for acylation in organic synthesis. Early literature reveals that traditional carbonates like phosgene triggered safety fears and environmental headaches. By the late 1980s and early 1990s, inventors were hot on the trail of more benign options—ones that packed efficiency without the same toxic baggage. Once Di(Succinimido) Carbonate rolled onto the scene, the tide changed in peptide chemistry and pharmaceutical process development. What started as a niche reagent now finds itself referenced across academic papers diving deep into efficient amide bond formation and linker strategies for new drugs.
Di(Succinimido) Carbonate doesn’t dress itself up. It’s a no-nonsense reagent famous among chemists for activating carboxylic acids, making life easier in peptide synthesis and bioconjugation. Your typical sample shows up as a white or pale yellow powder, stable enough to hold up short-term on the bench but best stored away from any hint of moisture. Industry suppliers usually ship it in UV-protected bottles or sealed foil to keep it from picking up water, which would lower its reliability. Product datasheets usually focus on its role in introducing N-hydroxysuccinimide esters—favorite handles for gentle, selective coupling—rather than get lost in marketing hype.
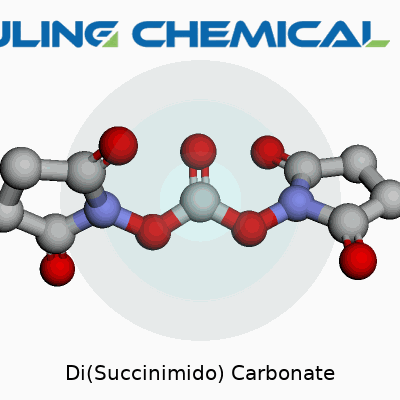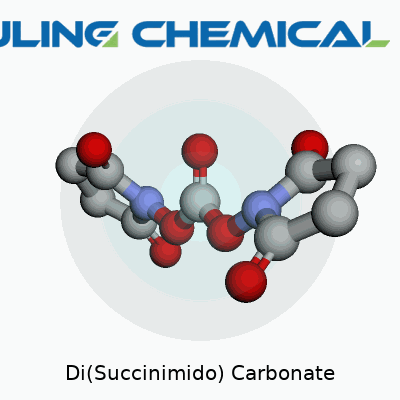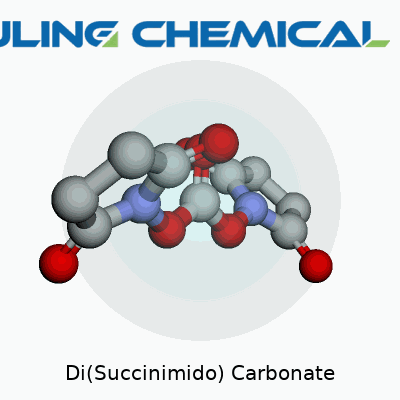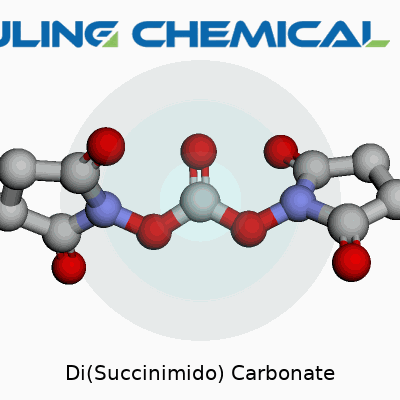
This carbonate doesn’t try to surprise you. Its molecular formula reads C9H8N2O7 and a molecular weight clocks in at about 256 grams per mole. Its melting point hangs above 100°C, so it’s not the sort of solid that melts from idle hands. Solubility in organic solvents like DMF, DMSO, and acetonitrile stands moderate; you won’t see much luck if you dump it straight into water, since it hydrolyzes away. Structurally, it features two succinimido groups flanking a central carbonate, which gives it that reactivity prized in chemistry. The compound packs enough stability to ship and store without fuss, yet springs to action under mild conditions once it’s in the right company.
Certified batches of Di(Succinimido) Carbonate usually hit purity marks above 98% by HPLC. Industry labeling highlights its sensitivity to hydrolysis, urging users to open containers in dry boxes or under dry air. You won’t see it paired with strong acids or bases on safety sheets—water and heat top the list of things to avoid. Container sizes range from small vials (for research) to kilo packs (for pilot plants), and labels always carry hazard information about skin or eye contact, with a warning for respiratory sensitization. Proper UN numbers and GHS pictograms appear prominently so everybody in the handling chain stays clued in. Material Safety Data Sheets feature first aid measures and storage best practices up front to foster a culture of lab responsibility.
Manufacturers produce Di(Succinimido) Carbonate by reacting phosgene or phosgene equivalents with N-hydroxysuccinimide under strictly controlled temperatures and inert conditions. The process demands a dry atmosphere to avoid side reactions, and careful purification via recrystallization or column chromatography yields a clean product. Large-scale setups prioritize minimum exposure to phosgene derivatives, swapping in safer alternatives where possible. Site managers invest heavily in ventilation, containment, and downstream processing to keep both product quality and workplace safety strong.
This carbonate’s main claim to fame involves activating carboxylic acids to produce N-hydroxysuccinimide esters, which then couple efficiently to primary amines. That makes it a key ingredient in both peptide synthesis and life sciences research. Additives like DMAP (4-dimethylaminopyridine) often boost reaction rates, but the core chemistry leans on the carbonate’s ready transfer of an active ester. Beyond standard coupling, chemists have modified the carbonate scaffold, bringing in substituents or changing the leaving group to tweak selectivity or solubility. Each time someone designs a new linker or cleavable group for drug delivery, the backbone of Di(Succinimido) Carbonate often inspires their route.
You’ll see Di(Succinimido) Carbonate called by several names depending on the supplier or protocol. Some catalogs list it as Disuccinimidyl Carbonate or DSC. Older literature sometimes refers to it by its systematic IUPAC name, which is unwieldy but thorough. Across language barriers, trade names emerge, but the chemistry world gravitates toward DSC for shorthand in papers, patents, and presentations, reinforcing a sort of street name that keeps communication brisk.
Anyone passionate about lab safety will respect the protocols that come with DSC. Workplaces require gloves, eye protection, and powder-only handling under dry air. Labs install local exhaust ventilation and train staff on spill response because the powder can sensitize skin and mucous membranes. Regulatory standards like REACH and OSHA provide guidelines for limiting exposure and recordkeeping. Eye wash stations and chemical showers must be within a quick walk. Employees report incidents without fear of retribution, and suppliers must provide clear, comprehensive hazard information along with the product.
DSC became a staple in peptide and oligonucleotide synthesis, but its reach stretches much farther. It’s the go-to for preparing active esters in the development of bioconjugates: antibody-drug conjugates, enzyme-protein labeling, cross-linking reagents, and surface modification of chromatography supports. The biomedical field values its mildness—reactions run at room temperature, protecting sensitive biomolecules from denaturing. From my own welter of lab notebooks, half the efficient one-pot coupling reactions banked on DSC to keep yield high and byproduct profiles low. With more targeted drugs and diagnostics flooding the research pipeline, DSC’s role keeps growing.
Academic groups keep exploring ways to boost the selectivity, speed, and utility of DSC in challenging syntheses. One busy area looks at swapping out the standard succinimide groups for others with tunable reactivity, aiming to improve coupling in peptide chains that cause trouble with aggregation or side reactions. Others work on integrating DSC-like reagents in flow chemistry or green manufacturing, shaving off solvent volumes or finding water-tolerant reaction protocols. Industry partners team up with universities to design custom DSC analogs for bioorthogonal chemistries—especially in site-specific drug modifications—fueling patent portfolios that widen the compound’s reach.
Toxicological studies on DSC focus mainly on acute skin, eye, and respiratory hazards. Animal testing shows that ingestion or high-concentration exposure causes local irritation, but systemic toxicity remains lower than legacy compounds like phosgene derivatives. Chronic exposure, as far as published studies go, does not significantly increase carcinogenic, mutagenic, or reproductive risk, though prudent lab practice still restricts duration and frequency of handling. Waste disposal guidelines treat spent solutions and rinses as hazardous, funneling waste for incineration or specialist treatment. Gaps remain in the environmental fate data—future research should clarify any breakdown products in water or soil, especially as production volumes rise.
Future applications for DSC lie both in deeper bioconjugation strategies and in replacing less selective or more hazardous coupling reagents. Peptide therapeutics and antibody technologies rely on reliable linkage chemistry as molecular weights soar and patent claims grow more complex. If researchers succeed in engineering water-stable DSC analogs, new classes of biosensors or direct aqueous-phase conjugates may emerge. Industry’s push for greener processes could inspire innovative recycling or recovery methods for DSC byproducts, shrinking the environmental footprint. As regulatory standards tighten, expect pharmaceutical and biotech developers to keep steering toward reagents like DSC that marry selectivity, safety, and process simplicity.
Di(Succinimido) Carbonate, usually called DSC in scientific circles, often gets overlooked by folks outside labs. At first glance, it sounds like just another complicated chemical, but it pulls a lot of weight for researchers and manufacturers. DSC works as a coupling agent. Its job is to help connect molecules together, especially in areas like pharmaceuticals and biochemistry.
Take medicine development. Scientists use DSC to link building blocks called amino acids, forming peptides. Many life-saving drugs, diabetes treatments, and even some cancer therapies rely on these peptides. DSC steps in to kick-start these connections, doing it cleanly and more efficiently than older reagents. That efficiency cuts down on waste and unwanted byproducts, a key demand now that pharmaceutical factories watch every gram of raw material. The push toward greener chemistry taps into this aspect of DSC, since less mess also means easier purification. Patients and doctors sometimes assume pills magically appear on pharmacy shelves; in reality, the smooth linking of molecular chains makes all the difference in production timelines and patient safety.
Researchers working in protein and antibody science have gotten a boost from DSC, too. Labeling and modifying proteins is easier with this compound, letting scientists track how proteins behave during experiments. Once a scientist can map these interactions, it opens doors for new diagnostic tests and targeted therapies. Precision matters whenever research shifts quickly from bench to bedside.
Working with any chemical calls for respect and preparation. DSC doesn’t rank among the harshest in the lab, but its reactive nature requires gloves, goggles, and good ventilation. By focusing on careful handling, labs keep people safe and protect valuable experiments from contamination.
So why care about a substance buried in technical papers? Reliable building-block chemistry forms the backbone of new treatments, biotech research, and industrial efficiency. Every time a university group or a small startup brings a new treatment up for trials, they bet on tools like DSC to help reach a breakthrough before funding dries up.
Recent demand for personalized medicine and rapid vaccine development put pressure on labs to adopt smarter, faster reagents. DSC stands out for its relative ease of use, cost-effectiveness, and the clear benefits it brings to process chemistry. By supporting smoother synthesis and reducing purification headaches, the compound lets scientists spend less time troubleshooting and more time pushing for results that matter to patients.
Suppliers should keep transparency high regarding DSC’s sourcing and purity. Open communication between chemical manufacturers and users helps speed up problem-solving and trust. Teamwork across the supply chain ensures quality and gives researchers more confidence in their results—especially as stricter regulations roll out across drug manufacturing worldwide.
As industries hunt for sustainable solutions and cleaner processes, compounds like DSC offer encouraging steps forward. Sometimes the unsung chemicals hold the greatest influence, shaping future discoveries before products ever see daylight on store shelves or pharmacy counters.
Di(Succinimido) carbonate, often abbreviated as DSC, keeps turning up in peptide chemistry circles. Its formula, C7H6N2O5, sums up a carbonyl group linked to two succinimide rings. These rings make all the difference in how researchers turn amino acids or peptides into useful building blocks.
Sketching the molecule, you’ll see the carbonate central group double-bonded to oxygen and linked by single bonds to two succinimide moieties. Each succinimide holds a five-membered ring with two carbonyl groups facing outward, bringing stability and reactivity to the molecule.
Those two succinimide rings aren’t just along for the ride. They create a balance: tough enough to keep their shape under routine handling, but reactive enough to swap out for other partners during bioconjugation reactions. I remember my first work in the lab using DSC — trying to activate a simple alcohol on a peptide chain. DSC made the process smoother than other carbonate options that easily fell apart or produced byproducts I couldn’t separate.
One key function stems from the placement of the succinimido groups. Their geometry lets them serve as great leaving groups. That means, when you throw in nucleophiles like amines, the succinimide slips away to make room for useful bonds without dragging in lots of side reactions. Labs appreciate this, since fewer steps and less cleanup add up to lower costs and higher purity.
DSC finds its main use in pharmaceutical settings, peptide synthesis, and biochemistry research. Peptide coupling comes to mind first. By activating hydroxyl or amino groups, DSC can get peptides and proteins to link up without needing tough reagents or harsh conditions. I’ve also seen it crop up in cross-linking reactions — like adding a linker arm between two proteins without losing their function. Its balanced reactivity fits applications where you need delicate, controlled outcomes.
The pharmaceutical world relies on molecules like DSC for the production of drug conjugates, targeted delivery systems, and diagnostic probes. If you’re working in a protein-modification lab or crafting new molecular probes, you’ll likely come across DSC sooner or later.
Peer-reviewed publications, including studies from the Journal of Organic Chemistry and peptide research literature, show consistent data on DSC. Its recognized structure ensures reproducibility across labs, which is huge when troubleshooting an experiment or cross-referencing protocols. Take proper handling seriously, though — DSC reacts to moisture, and improper storage will yield unreliable results. Sigma-Aldrich, Merck, and other chemical suppliers provide high-purity DSC, keeping batch variation low.
Production cost and safety keep coming up as sticking points. While DSC offers clean reactions, its synthesis relies — at least for now — on starting materials and methods that might not always align with green chemistry goals. Process chemists work to tweak conditions: aiming for higher yields, reducing toxic byproducts, and finding alternatives that cut down on waste. Scalable, eco-friendly synthesis methods would go a long way, especially as demand rises in pharma and biotech.
Researchers also eye improvements in shelf stability and packaging. Building a supply chain that reduces exposure to water and heat could save labs from ruined stock and costly delays. Sustainable packaging is a rising concern as well, echoing wider environmental priorities.
Tools like DSC play a major role in shaping how scientists connect biomolecules, engineer new drugs, and push the boundaries in life sciences. Getting the structure right is just one step. Making its production, distribution, and on-bench performance sustainable and safe remains a shared goal for chemists and the pharmaceutical industry alike.
Anyone who has ever spent long hours in a lab knows the feeling well: A mishandled reagent can ruin equipment, spoil research, or even threaten health. Di(Succinimido) Carbonate calls for clear thinking because storing it badly does more than waste money—it opens the door to trouble. From what I’ve seen, safe storage comes down to three key conditions: temperature, container choice, and awareness.
Di(Succinimido) Carbonate keeps best in a cool, dry spot out of direct sunlight. Excess heat or damp environments break down the compound faster, and that means less reliability for anyone counting on purity. Most chemistry references suggest a storage temperature between 2°C and 8°C. In practice, that often means a dedicated fridge. At a large research institute where I once worked, we lost an entire stockroom batch to summer heat waves because nobody bothered checking the thermometer. That mistake is hard to forget—the replacement cost alone gave the purchasing team a headache for weeks.
Every time a container opens, moisture and air can find their way in. Both these things encourage clumping or chemical changes you simply can’t fix later. Amber glass bottles with tight screw caps work well for small or medium amounts. The glass provides a solid barrier without reacting or leaching, while the dark color blocks ultraviolet rays. Some facilities also use lined aluminum cans for bulk amounts. I’ve worked with plastics in the past, but glass or lined metal outlasted them by far, especially during storage over six months.
Label every bottle with the date received, the expiry date, and the last time it was opened. This habit prevents the classic mistake where an old, half-used bottle gets mistaken for a fresh one. One morning, a lab partner grabbed an expired batch for a peptide synthesis, and the reaction gave ghost results. That kind of preventable error rattles trust throughout a team. Keeping similar-looking bottles of other carbonates separate reduces confusion, too. Store this compound on an upper shelf away from acids and bases. Mixing even small amounts by mistake can risk unwanted reactions and wasted hours cleaning up spills.
Well-ventilated storage offers another layer of safety. If fine dust or vapors escape, a proper airflow setup prevents them from lingering where people work. Filtering hoods aren’t extras—they limit long-term exposure. At my favorite teaching lab, standard practice put absorbent mats under all storage areas, so leaks or spills wouldn’t find their way into cracks or drains. A clean, dry workspace can make a nasty situation a short-lived one instead of a disaster.
Every step above means less risk, stronger results, and fewer headaches. Mishaps in chemical storage leave lasting lessons. Double-check the temperature, keep bottles tight and labeled, and build habits for safety. Companies like Sigma-Aldrich, who set supplies and guidance worldwide, offer clear storage instructions—there’s no shame in following expert advice when lives, research budgets, and reputations are on the line.
Di(Succinimido) Carbonate often pops up in chemistry labs, especially in pharmaceutical research, thanks to its role in peptide synthesis. Since this compound isn’t as famous as acetone or bleach, folks might wonder if their curiosity is really full of risk. In labs, safety manuals usually treat all carbonates with a big dose of caution, but digging into the data helps separate real threats from myth.
I’ve handled Di(Succinimido) Carbonate for peptide projects. The first thing any decent supervisor tells you is to respect chemicals you work with. I have never seen this compound cause a big incident, though any careless handling demands a stern lecture. This carbonate carries a mild irritant status. If it lands on your hands, you’ll probably feel some discomfort or itching, so gloves become more than just compliance—they’re habit. Fumes aren’t usually an everyday worry, but dust from the solid does trigger sneezing fits if you don’t work in a fume hood.
Toxicity always scares people, probably because the science behind it sounds like something only experts can truly grasp. With Di(Succinimido) Carbonate, things look less severe than some other reagents in the same lab. Research and safety sheets mention low acute toxicity. It does not get absorbed easily through the skin, and swallowing it accidentally would mostly lead to stomach upset, rather than any major poisoning. The LD50 (lethal dose for 50% of test animals) tells a lot about danger. In animal studies, this carbonate lands in a relatively mild zone, far less toxic than pesticides or heavy metals.
Fears over long-term exposure sound more intense than what’s supported by published evidence. So far, there is no public record showing Di(Succinimido) Carbonate causing cancer or severe chronic health damage in humans. It doesn’t act like a heavy toxin in cell studies either. Still, it deserves careful handling since powder inhalation can be unpleasant, and repeated skin contact never does anyone any good.
Gloves and goggles serve as a simple shield. Fume hoods offer a second layer of comfort for those who don’t want to sneeze through the shift. Getting this stuff in your eyes or nose will never be fun. Lab culture builds around respect for chemicals, and tools like spill neutralizers and safety showers prove their worth if an accident happens.
You don’t have to go overboard and treat Di(Succinimido) Carbonate like an outlawed substance, but shrugging off the risks doesn’t reflect wisdom, either. If anyone at home stumbles upon this compound (maybe bought as part of a chemistry kit), safe storage and solid labeling keep families and pets out of trouble.
Factories and schools that use Di(Succinimido) Carbonate should lean into solid training for newcomers. Clear charts, simple emergency instructions, and visible personal protective equipment lower risk for everyone. Good waste management makes a real difference—no rash dumping down the drain. Following local regulations about chemical disposal always lands on the smart side of history.
Talking about risks openly, using facts, and sticking to habits built on experience builds trust not just within a single lab, but in the wider community. Being honest about the limits of hazard can prevent overreaction or carelessness, letting research and production carry on with fewer hurdles and fewer hazards.
Every seasoned chemist knows some of the best reagents in the lab aren’t flashy. Di(Succinimido) Carbonate, or DSC, proves this. Sitting in brown bottles on benches, it pulls weight in ways you won’t find in textbooks that dwell on blockbusters like palladium catalysts. My own early work involved making simple peptides, but without the clean activation DSC gives, I’d still be scraping failures off glass columns. The real power of DSC in research and production comes from how it makes tough jobs easier, not from being mysterious.
Ask researchers making peptides why they trust DSC. The answer centers on safety and reliability. Compared with more toxic reagents, such as phosgene or carbonyldiimidazole, DSC skips the hazardous fumes and sticky byproducts. In process chemistry, this matters. I’ve seen colleagues struggle with impure batches made from tricky coupling agents, only to switch to DSC and breeze through synthesis with purer end results and fewer headaches.
The dry language of journals hides genuine excitement when a reagent actually works every time. DSC can activate carboxyl groups on amino acids, which lets scientists stick them together in a controlled way. The reaction’s regularity means it translates well from small-scale academic projects all the way up to pilot plants working on new therapeutics. This kind of connectivity means researchers don’t get tripped up by scale: what worked in a glovebox keeps working when gallons are on the line.
With modern drugs merging proteins, sugars, and small molecules, bioconjugation tech has never been more valuable. DSC shines here. It links amines to carboxyls on proteins and polymers, letting drug makers design antibodies or sensors meant to do one job, without dragging along side reactions. I remember a project where conjugating a drug to a protein used to take days and sometimes led to clouds of gunk. Using DSC trimmed hours off protocols, with almost no mess.
This clean reactivity means better data. Whether preparing a fluorescent tag or coupling a polymer for drug delivery, results are more reproducible and less likely to spook regulatory teams who worry about structural unknowns in pharma production.
In the past, a reaction’s waste stream got ignored. These days, industry teams—myself included—try to cut toxic byproducts and tough cleanups. DSC doesn’t just ease concerns; it also shortens purification routines, cutting down solvent use and disposal costs. I’ve seen protocols that swap out classic, dangerous coupling chemicals for DSC, yielding products with less work and a lighter regulatory burden.
Supporting this, a handful of publications and process patents back up what researchers see on the bench. Teams at big pharma companies have moved over to DSC for high-value steps, and academic groups recommend it for teaching labs and green chemistry tracks. This matches my experience. If a graduate student wants a result they can replicate—without making the lab smell like warfare—they grab DSC.
Beyond making drugs, DSC helps produce better polymers and functional materials. The same chemistry that delights peptide scientists applies. It crosslinks polymers gently, controlling texture and function in biodegradable plastics or medical adhesives. My work intersected with an engineering group testing new hydrogels for wound care. The difference DSC made showed up under the microscope: cleaner structures, predictable mechanical strength, and fewer surprises that spooked both the scientists and the end users.
Applications of DSC reflect trust built from safe handling, consistent chemistry, and fewer surprises. I’ve seen how a good reagent, backed by real-world experience and clear regulatory records, moves out of narrow research lanes and starts to appear in medicines, devices, and even classrooms. Folks in chemistry and industry stay sharp by adapting tools that just work—and on that list, DSC carves out its own place.
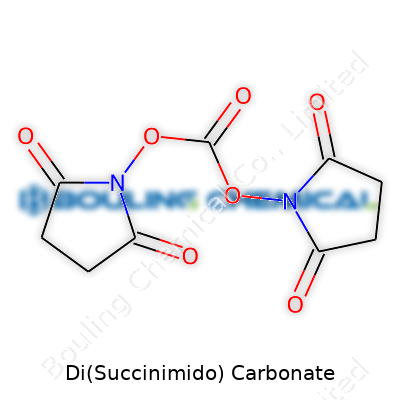
| Names | |
| Preferred IUPAC name | Disuccinimidyl carbonate |
| Other names |
DSC Disuccinimidyl carbonate |
| Pronunciation | /daɪˌsʌk.sɪˈnɪmɪ.doʊ ˈkɑːr.bə.neɪt/ |
| Identifiers | |
| CAS Number | [74124-79-1] |
| 3D model (JSmol) | `3DModel:JSmol:C1(=O)N2CCCCC2C1=O.O=C(OCOC(=O)N3CCCCC3C4=O)C4=O` |
| Beilstein Reference | 3703020 |
| ChEBI | CHEBI:132962 |
| ChEMBL | CHEMBL4201802 |
| ChemSpider | 21361241 |
| DrugBank | DB11262 |
| ECHA InfoCard | 03c4f39f-5d2e-4877-9f37-6384e97f2580 |
| Gmelin Reference | 112075 |
| KEGG | C18806 |
| MeSH | D023315 |
| PubChem CID | 12504 |
| RTECS number | FF9280000 |
| UNII | D173M78TRQ |
| UN number | Not regulated |
| CompTox Dashboard (EPA) | DTXSID70874546 |
| Properties | |
| Chemical formula | C9H8N2O7 |
| Molar mass | 274.18 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.542 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -1.1 |
| Vapor pressure | <0.01 hPa (20 °C) |
| Acidity (pKa) | 13.2 |
| Basicity (pKb) | 11.5 |
| Magnetic susceptibility (χ) | -65.0e-6 cm³/mol |
| Refractive index (nD) | 1.508 |
| Viscosity | Viscous liquid |
| Dipole moment | 6.7284 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 242.8 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | V03AB53 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07, GHS05 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P304+P340, P312, P321, P332+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| NIOSH | WTW7540000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 mg/m³ |
| Related compounds | |
| Related compounds |
N-Hydroxysuccinimide Disuccinimidyl suberate Disuccinimidyl tartrate Bis(succinimido) glutarate Disuccinimidyl adipate |