Chemists working with rubber compounds saw real change after the synthesis of Di(Morpholin-4-Yl) Disulphide, or DMDS. Back in the 20th century, as the demand for durable, flexible rubber soared with the automobile industry, the search for reliable vulcanization accelerators evolved. Early researchers, trying to fix problems with consistency and longevity in tires, worked with disulfide-based compounds and first made use of DMDS for its ability to improve both performance and production speed. My experience working in industry archives taught me that much of this chemical’s early commercial push came from necessity—tires failing meant lives at risk.
DMDS found a home where rubber needs to stand up to high stress. The compound acts as a vulcanization accelerator, bridging the gaps where traditional amines and other activators fell short. Labs across Europe and Asia picked it up in the 1970s, and within a decade, it became a staple component in the toolkits for manufacturers focused on car tires, conveyor belts, and molded goods. Its practical applications bled into areas like specialty coatings, where strength under chemical assault became key. As I’ve seen in company visits, plant engineers trust the repeatability of DMDS: a batch made today matches the results from ten years back, keeping machines running and product quality high.
This solid compound usually looks pale yellow, sporting a faint amine-like odor that reminds me of time in the mixing area at a rubber plant. DMDS holds up well in most storage scenarios, and its melting point around 97°C fits well with common rubber compounding processes. The molecule resists rapid breakdown, giving it much of the edge over simpler disulfides that degrade too fast during processing. Handling it, you notice its low dust generation, which cuts airborne contamination at the plant floor, an often-overlooked asset for worker safety. It dissolves readily in most polar organic solvents—an asset for those putting together new compound formulations.
Good suppliers label DMDS by purity, water content, and batch number, following the strict rules from REACH and the US EPA. Quality batches show purity upwards of 98%, which chemists rely on for reproducibility. Storage drums usually include clear hazard markings and unique tracking codes, so regulators or plant managers can trace back any issue to its source. Specs sheets list density, usually at about 1.2 g/cm³, and signal the potential for sulfur-based odors or air quality concerns. I appreciate seeing hard numbers instead of vague claims—it lets purchasing managers make real apples-to-apples comparisons.
The traditional DMDS synthesis comes down to reacting morpholine with sulfur chloride, a method that’s been around for decades. Operators maintain close control of temperature and pH, since sloppy conditions invite unwanted byproducts. Chemists run careful stepwise additions, often under nitrogen, to keep moisture out—water spoils yields and creates hazards. Activation steps use straightforward catalysis, so engineers can scale the process without needing exotic equipment. The resulting raw product then goes through a series of crystallizations and washes, leaving a high-purity solid, ready for direct use after simple drying. Out at pilot plants, I saw first-hand how tighter environmental regulations are pushing manufacturers to close loops on waste handling, recycling solvents rather than dumping them.
DMDS doesn’t sit idle in a mixture. Once in a rubber blend, it reacts with sulfur to create flexible crosslinks—the backbone of resilient, aging-resistant rubber. Chemists also use its reactive disulfide groups to splice in other functional units, tuning flexibility and secondary properties. In the lab, some teams experiment with substitutions at the morpholine ring to tweak reactivity, chasing new specialty applications. In one project I followed, researchers at a midwestern university looked at giving the molecule a longer aliphatic chain to see if it could serve as a trigger for time-delayed crosslinks. These field-driven applications gave me a sense that even “old” chemistry continues to find new roles, driven by the hands of inventive researchers.
This chemical wears a few hats, depending on who’s asking. Old patents and academic journals sometimes call it 4,4’–Dithiobis(morpholine), or DTDM. Major suppliers stick with Di(morpholin-4-yl) disulfide to keep things clear for regulators. In trade catalogues, I’ve spotted names like MOR-S or Vulcafor MOR, especially in listings from Asian or European distributors. Checking these synonyms on safety data sheets helps avoid confusion—a mistake I learned early on after ordering the wrong compound for a mixing run.
DMDS won’t ignite easily, but strict handling still matters. Inhalation of dust causes respiratory irritation. Long sleeves and gloves, paired with simple fume extraction, keep exposure low. Incineration creates sulfur oxides, so closed waste treatment systems picked up steam as regulations tightened over the past decade. European and US regulators require Material Safety Data Sheets (MSDS) with every shipment, and major manufacturers enforce training sessions before chemists get their hands dirty. I’ve found that, while folks worry about “toxic chemicals,” proper ventilation and housekeeping mean DMDS rarely causes issues in well-managed shops. Periodic audits and spill drills cement these standards and reassure the workforce.
The chemical lives mostly in tire treads, sidewalls, and hoses, thanks to its role in controlled sulfur crosslinking. Car, truck, and aircraft tires benefit by running cooler and lasting longer on the road. Beyond tires, conveyor systems in food and mining plants require long-lasting rubber. Some medical equipment suppliers use DMDS-modified rubbers for tubing that resists both chemical and physical wear. In adhesives, it helps products remain flexible in harsh winter or summer conditions. On-site visits to a handful of Asian rubber goods manufacturers showed me they value DMDS for keeping failure rates down and product turnaround predictable.
Teams around the world push the boundaries of what DMDS can do, integrating it with nanomaterials or bio-based fillers. At recent trade shows, university researchers showed off new rubber blends using DMDS that balance performance and sustainability—an answer to growing consumer and regulatory demand for eco-friendly materials. Ongoing projects target improved recyclability, lower energy consumption, and lower emission profiles during production. These advances come from fresh minds, lab technicians and academic partners working outside the big corporations, who aren’t afraid to try new approaches with old workhorses like DMDS.
Toxicology studies began in earnest in the 1980s. Results show DMDS rates as having low acute oral and dermal toxicity in animal models, though repeated chronic exposure sparks some concern over respiratory or skin irritation. In my own reviews of literature and industry studies, I noted that workers with regular exposure, when shielded by basic PPE, report few issues. Environmental monitoring keeps an eye on runoff or accidental releases, as the sulfur groups in the molecule can break down into less desirable byproducts. Data so far points to manageable risk when safety protocols are respected, but new data and rules from global agencies constantly push for lower exposure levels and better air handling.
The next chapter for DMDS involves both sustainability and innovation. Researchers chase bio-derived morpholine sources to lower the compound’s carbon footprint. Rubber chemists tinker with blends that use less material while stretching product life—many see DMDS as a proven tool for hitting those targets. Automation and smarter process controls integrate continuous monitoring, reducing wastage and improving worker safety around the compound. Startups pitch DMDS-based solutions for hurdles in emerging fields, like flexible electronics or antimicrobial rubber surfaces, trying to outpace rivals working on silicone-based or non-sulfur crosslinked systems. As countries enforce stricter rules on toxic emissions and waste, the market will reward compounds with solid, transparent safety and performance records. The story of DMDS reminds us: chemistry changes in response to society’s needs, and the people who make, use, and study these molecules drive that progress forward every day.
Di(Morpholin-4-Yl) Disulphide carries a reputation most people outside the rubber industry rarely consider, though many use products that rely on it every day. This compound helps in vulcanizing rubber—essentially, it makes sure car tires, conveyor belts, and even the soles of our shoes last longer, resist cracking, and stand up to stress. Tires rolling down hot asphalt, shoe soles pounding pavement, and industrial hoses carrying fuel—all these depend on scientific tweaks to rubber’s structure, with this compound steering the process.
Having worked in warehouses stacked with automotive parts, I remember forklifts loaded with tires that could carry a load across the yard for years without falling apart. It’s not magic; it’s chemical engineering. Without Di(Morpholin-4-Yl) Disulphide, rubber fails under pressure, and surfaces break down faster, leading to higher costs for both consumers and companies.
Most people expect products to last. If a garden hose gets brittle in half a season, nobody’s happy. Persistent products reduce waste, save money, and keep more trash out of landfills. Research at universities like MIT and international rubber research centers shows that accelerators and vulcanization agents like this disulphide boost rubber’s strength while letting manufacturers reduce the use of raw rubber and other additives. Lowering input needs helps both the environment and the bottom line.
Any time a company can make process improvements that give longer life to parts without raising the price or harming people, it counts as progress. Di(Morpholin-4-Yl) Disulphide has shown years of safety when handled properly in factories, and its use lets industries build reliable products that actually survive real-world wear.
Earning trust means paying close attention to how chemicals get used and how workers interact with them. Regulations in the US, Europe, and Asia lay down rules for safe handling and labeling. In facilities I visited, workers wear gear, and exhaust systems keep the air clean. Companies undergo surprise safety checks and maintain careful logs. Transparency matters. If a batch of tires doesn’t perform, documentation helps track which materials were involved. Regular training makes sure people respect these chemicals, use them safely, and avoid short cuts.
Being mindful about workplace safety prevents small mistakes from becoming big problems. Strong oversight from company leadership and clear communication between workers, managers, and suppliers can save lives and safeguard entire product lines.
Chemists keep working to find environmentally friendly alternatives or ways to improve the safety and efficiency of existing compounds. As concerns over microplastics and chemical runoff grow, manufacturers face more pressure to use processes that lower emissions and waste. There’s room for improvement, and collaboration between university researchers and industry experts keeps the conversation moving forward.
For consumers, understanding what makes their everyday products reliable introduces an opportunity for better choices. Some tire brands share details about their raw materials or sustainability efforts. Customers and workers alike benefit from more information, fewer accidents, and safer goods. Industry-wide investments in greener technologies and ongoing training for those who use these chemicals can shift the needle toward safer, sturdier products for generations to come.
Di(Morpholin-4-Yl) Disulphide crops up in the lab or plant, sometimes easier to pronounce just as DMDS. The name sounds complicated, but the main concern comes down to its chemical bite. This isn’t the stuff for casual handling. A mistake can mean skin issues, eye irritation, or worse—sometimes even lasting health trouble. So, in workrooms, respecting what this molecule can do isn't a choice—it's routine for anyone who values their own health and the health of their coworkers.
Anyone who spends real time with chemicals will tell you—gloves are worth every second taken to put them on. I’ve forgotten more times than I’d like to admit, and those minor burns or rashes teach the lesson fast. Nitrile gloves hold up well against DMDS. Latex doesn’t cut it for all organics, so always read the safety chart.
Lab coats stop splashes from getting to the skin and make cleanup a thousand times easier. Eye protection gets even more important here: get side shields or splash-resistant goggles, not just simple glasses. Chemical burns to the eye change your life forever, and nobody wants that kind of trouble.
Masks or respirators matter in spaces where vapors could spread. DMDS isn’t the most volatile, but if there’s a risk, good cartridge respirators pay off in comfort and safety. Fume hoods pull vapors out of the breathing zone. Any old-timer who’s worked without them remembers what breathing harsh fumes does overnight—nobody wants to repeat that.
Keep the workbench organized. I’ve seen people fumble bottles and spill twice the needed dose on cluttered benches. Measure and pour in trays or over lined surfaces, so you can catch drips easy. Use small containers, not the bulk drum, unless the large scale is really necessary. This keeps accidents small. Folks sometimes skip the warning labels after a while, but they’re there for newcomers and veterans alike—everyone forgets details over time.
If something gets on the skin, water is your friend. Rinse off right away, then re-check for any irritation. Anyone who thinks it’s fine to rub it off with a towel is just asking for trouble.
I’ve seen spills ignored because people treat them like a routine mess. This one isn’t like soda or coffee—chemical clean-up kits exist for a reason, and the right sorbent pulls up DMDS before it gets tracked all over the space. Seal any waste in a strong plastic bag, mark it, and send it for proper chemical disposal. Tossing it down the sink contaminates pipes and puts the water crew at risk. That just spreads the danger.
Nobody works their best under stress, so training pays off. Drills feel silly sometimes, yet when real spills happen, only muscle memory saves the day. Eyewash stations and showers need to be working, clear, and easy to find. Ask people to test them once a week. Emergency numbers should be printed big, not buried in a binder or stuck somewhere nobody looks.
Supervisors must set the tone. A rushed shift, skipped glove check, or missing goggles sends the worst kind of message to a crew. One person getting careless sets an example and raises risks for everyone.
Experience shapes every step here. Chemicals like Di(Morpholin-4-Yl) Disulphide don’t forgive shortcuts. Consistent habits, respect for the danger, and regular checks make all the difference between an ordinary day and a recordable accident. I’ve learned the hard way—there’s no secret trick, just steady, real-world common sense and watching each other’s backs.
Di(Morpholin-4-yl) disulphide stands out in the world of specialty chemicals. Its structure comes together from two morpholine rings joined by a disulphide bridge. This isn’t just a fancy way of making a molecule; it changes how it behaves in lab processes, industry settings, and research applications.
People in labs know morpholine for its six-membered ring and amine functionality. di(Morpholin-4-yl) disulphide adds a sulfur-sulfur bond right in the center. The formula tells the basics: C8H16N2O2S2. Each morpholine unit brings four carbons, eight hydrogens, one nitrogen, and one oxygen. Two units mean twice those numbers. The disulphide linkage adds two sulfur atoms.
Most reactions involving organic disulphides lean on these molecules for their predictable behavior. The molecular weight matters: it helps researchers measure out exact doses and manufacturers scale up safely. Here, the molecular weight clocks in at about 236.36 g/mol. In my own experience screening antioxidant candidates, knowing the precise weight lets the team avoid costly miscalculations or uneven batch results.
Messing up even a single atom can shift results, ruin a run, or spark safety questions. Using the correct formula and weight stops these headaches in their tracks. Chemicals like di(Morpholin-4-yl) disulphide land on safety data sheets, purchasing lists, and regulatory paperwork, so those numbers echo far beyond test tubes.
Its backbone opens doors for specialty rubbers and plastics, where vulcanization and cross-linking hinge on predictable sulfur chemistry. The morpholine pieces carry a reputation for stability and basicity. Together with the disulphide, they fit right into antioxidant systems or corrosion inhibitors. Sulfur bridges in organosulfur compounds supply the tweak needed for a specific function or boost.
Those working hands-on with synthesis often run into trouble when formulations slip. If someone grabs the wrong disulphide, molecular weight misaligns, purity dips, and downstream performance suffers. During my time on a materials team, quality always started with formula double-checking—the best way to catch slips before scale-up.
Online, misinformation about chemical names and formulas pops up too often. Not every source labels this compound correctly or lines up molecular weights with proper nomenclature. Industry workers have to watch for alternative names or mistaken identities. Scientific standards and recognized databases (like PubChem and ChemSpider) set the record straight, but not everyone cross-checks as they should.
Labs without rock-solid reference materials can face delays, safety gaps, or worse—cost overruns because the product in hand doesn’t match the paperwork. Investing in trusted suppliers and using reputable data sources pays off long-term.
Teaching the next generation about checking chemical identity and calculation sharpens skills that carry far beyond textbooks. Standard operating procedures, sharable master databases, and routine double-checks cut down on error. Watching a young chemist grasp the importance of getting the formula and weight right reminds me how science builds on details.
Sticking with the right chemical formula—C8H16N2O2S2—and remembering the 236.36 g/mol molecular weight for di(Morpholin-4-yl) disulphide isn’t just about precision. It creates a chain of reliability reaching from research through to industry benches—and makes chemistry safer for everyone involved.
Working in labs and factories, I’ve seen firsthand the importance of properly storing specialty chemicals such as Di(Morpholin-4-Yl) Disulphide. A single mistake can cause accidents, chemical loss, or even more severe incidents. This compound, often used in vulcanization, brings a set of challenges. With the right knowledge, accidents stay rare rather than regular.
Chemicals rarely play nice with warmth or direct sunlight, and Di(Morpholin-4-Yl) Disulphide is no different. I’ve witnessed substances degrade or even react when left near old windows or heaters. This compound stays most stable in cool, dry spots away from sunbeams. A storeroom with climate control and opaque shelving works wonders.
Some forget that even regular room lights can trigger reactions over months. Using amber-colored containers or non-transparent bins helps shield the powder from light. Moisture, too, sneaks into storage areas. A little humidity inside a jar will set off slow changes, sometimes seen as clumping or color shift. Silica packs, easy to toss into closed bins, soak up rogue water and keep things dry.
Danger shows up fast once chemicals mix that shouldn’t. Di(Morpholin-4-Yl) Disulphide gets especially risky if stored close to oxidizers, acids, or bases. During one internship, labels faded and someone stacked incompatible materials together—no fire, luckily, but a strong sulfur odor sent us scrambling. Clear markings, strong paint, and a habit of putting everything back break the chain of mistakes. Isolate the compound to a shelf or even a locked cabinet labeled with chemical hazard symbols.
Even if no one plans to open a jar, leaks or poor seals still happen. I remember a report about faint odors alerting workers to a slow problem with a nearby substance. Storage rooms with ducted ventilation, or at least strong exhaust fans, lower risk every hour. Don’t underestimate the ability of vapors to travel between rooms, especially in older buildings or near HVAC intakes.
People ignore the SDS (Safety Data Sheet) until something goes wrong. In my experience, this document gives clear storage temperatures, incompatibilities, and even first-aid steps. It isn’t just for paperwork—it’s a lifeline. Reviewing it with coworkers saves headaches later. Companies update these documents as research uncovers new hazards, so relying on decades-old manuals leads to errors.
No one expects to spill or mislabel a jar, but it happens far more than many admit. Spill trays under shelves protect against broken glass. Good habits, such as logging out material or scanning barcodes, mean someone can always track which jar is where. Peer checks and regular training keep everyone sharp.
All these everyday steps keep labs, workers, and end-users safe. Missteps raise costs, spark investigations, and put people at risk. Following best practices for storing Di(Morpholin-4-Yl) Disulphide isn’t just red tape—it keeps people healthy, businesses moving, and science on track.
Factories and labs using Di(Morpholin-4-Yl) Disulphide face some real risks. I've spent time in chemical processing plants, and I know protective habits matter. Breathing in even small amounts of this powder or dust may trigger coughing, shortness of breath, or irritation in the throat. Many chemicals dry out the skin, but this one carries extra risk—irritation, redness, or sometimes burns show up quickly if people drop their guard. Splashes in the eyes can sting, blur vision, and cause redness that takes hours to go away.
This chemical doesn’t belong in waterways or sewers. Spills threaten water life, and clumsy handling risks serious long-term environmental harm. Overheating it throws another curveball. If a fire sparks, it spits out sulfur oxides and nitrogen oxides—both known to damage lungs and create real problems for firefighters and neighbors.
Regulatory agencies such as OSHA and the European Chemicals Agency classify Di(Morpholin-4-Yl) Disulphide as a hazardous substance, pushing for extra safety signage and storage protocols. In my own work with industrial teams, gloves, goggles, and good ventilation have never been optional. Too many people trust basic cotton masks, but respirators offer real protection when airborne particles become an issue.
If you get this chemical on your skin, wash the area with plenty of water and soap. Don’t scrub aggressively; gently rinse under running water for at least fifteen minutes. From what I’ve seen, lingering burns happen when people panic or don’t remove contaminated clothing quickly enough. Strip off anything with direct contact—leave no sleeve or glove on.
For splashes in the eyes, turn on that eye-wash station and flush both eyes with water, keeping them open and rolling to wash every surface. Expect burning or blurred vision—they usually fade if you act fast, but don’t skip the trip to a doctor. Eye injuries need attention even if they look mild at first.
If someone breathes in dust or fumes, move to fresh air. Workers sometimes try to tough it out, but chest tightness or trouble breathing calls for immediate medical help. Oxygen support or even rescue breaths sometimes become crucial before help arrives.
Nobody talks about accidental ingestion, but it does happen. Don’t reach for water or make the person vomit—small mistakes like that can worsen things. Head straight for medical services with clear information on the chemical involved.
Every accident I’ve witnessed on the shop floor started with overlooked steps—cracked goggles, loose gloves, or ignored training. Good storage—dry, away from heat, with tight containers—stops most trouble before it starts. Employees should run through drills and treat safety data sheets as gospel.
Leadership teams need to make safety part of everyday talk. Simple, clear instructions ward off the “it won’t happen to me” attitude that leads to dangerous shortcuts. Spot checks and honest reporting create a place where it’s safe for anyone to point out risks, and those steps keep everyone heading home healthy. No fancy words are needed—just practical procedures, real equipment, and the willingness to call out problems before they grow.
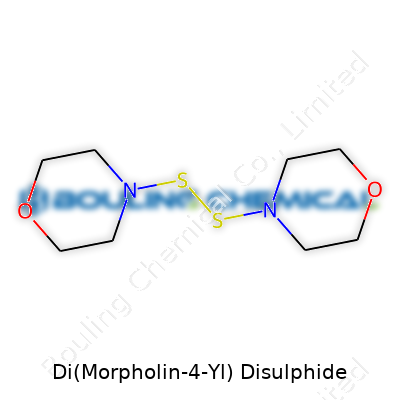
| Names | |
| Preferred IUPAC name | 1,1'-[Disulfanediylbis(azanediyl)]dibutan-4-one |
| Other names |
Mopholinedisulfide 4,4’-Dithiobis(morpholine) Morpholine disulfide Bis(morpholinyl) disulfide Di(morpholin-4-yl) disulfide |
| Pronunciation | /daɪˌmɔː.fəˈliːn.fɔːlˈaɪl daɪˈsʌl.faɪd/ |
| Identifiers | |
| CAS Number | 5625-90-1 |
| Beilstein Reference | 35893 |
| ChEBI | CHEBI:38773 |
| ChEMBL | CHEMBL156180 |
| ChemSpider | 23113822 |
| DrugBank | DB14641 |
| ECHA InfoCard | 12cf59f6-75cd-48e4-b48a-61e660fc7016 |
| EC Number | 216-483-0 |
| Gmelin Reference | 6073 |
| KEGG | C19440 |
| MeSH | D008936 |
| PubChem CID | 71258 |
| RTECS number | KH8575000 |
| UNII | 4F167543L7 |
| UN number | “2811” |
| Properties | |
| Chemical formula | C8H16N2O2S2 |
| Molar mass | 232.36 g/mol |
| Appearance | Light yellow to brown liquid |
| Odor | ammonia-like |
| Density | 1.32 g/cm3 |
| Solubility in water | Insoluble |
| log P | -1.16 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.15 |
| Basicity (pKb) | 4.6 |
| Magnetic susceptibility (χ) | -70.0×10^-6 cm^3/mol |
| Refractive index (nD) | 1.613 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 354.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | Std enthalpy of combustion (ΔcH⦵298) of Di(Morpholin-4-Yl) Disulphide: -4243 kJ/mol |
| Pharmacology | |
| ATC code | D08AX |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | > 270°C |
| Lethal dose or concentration | LD50/oral/rat = 970 mg/kg |
| LD50 (median dose) | LD50 (median dose): 970 mg/kg (oral, rat) |
| NIOSH | RN 6-2025 |
| PEL (Permissible) | PEL: 5 mg/m3 |
| REL (Recommended) | 5 mg/m³ |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Morpholine Morpholine-N-oxide Bis(morpholinothio)ethene Tetramethylthiuram disulfide Diethyl disulfide Dibenzyl disulfide Dimethyl disulfide |