Rubber didn’t always bounce back like it does today. Factories in the early 20th century faced limits with both flexibility and lifespan of rubber goods, watching as tires cracked and conveyor belts wore thin before their time. Research teams searching for a boost to the curing process stumbled across the benzothiazole family—chemicals with a knack for lending strength to rubber. In the 1920s, the discovery of Di(Benzothiazol-2-Yl) Disulphide offered a real breakthrough. Chemical companies and tire manufacturers quickly realized that this compound allowed rubber vulcanization to advance at lower temperatures and reasonable speeds. Di(Benzothiazol-2-Yl) Disulphide became a cornerstone in tire making and found its way into every shop that molded or extruded rubber, helping build the modern era of transportation and industrial mechanics.
Getting to know Di(Benzothiazol-2-Yl) Disulphide by name alone does little justice to its value. This organic compound belongs to the class of accelerators—chemicals that make the sulfur vulcanization of rubber much more efficient. It steps in and shortens the cooking time, lowers the heat required, and lets manufacturers hit precise mechanical properties tailored for each use-case. Factories moved away from old-fashioned, time-consuming methods because this accelerator slashes hours off the clock, reduces energy waste, and hands them consistent results. In practice, this means tires last longer, hoses don’t fail under pressure, and gaskets seal tight where it counts.
The compound appears as a light yellow powder, easy to handle, and with a faint odor. Unlike some more volatile chemicals, it doesn’t catch fire easily under standard shop conditions, which adds a layer of reassurance for process operators. Solubility in water stays close to zero, cutting down the risk of it leaching through soil or drains if a spill ever occurs. Its melting point sits above the boiling point of water, holding up through tough processing steps. Di(Benzothiazol-2-Yl) Disulphide dissolves in solvents like acetone and benzene, making it compatible with industrial resin blending and surface mixing processes.
Quality managers in rubber plants look for tight spec sheets before ordering a batch. A typical product guarantees a content above 96%, with moisture levels kept below 0.3% and an ash percentage no higher than 0.3%. This ensures minimal impurities or interference during mixing. Labels on shipping drums flag hazard precautions, UN proper shipping name, and recommend storage at room temperature, away from oxidizers or flammable stock. Clear batch coding and production dates support traceability—no small matter when defects or off-spec material could mean a costly recall.
Lab technicians approach synthesis by coupling 2-mercaptobenzothiazole (MBT) under oxidizing conditions. The process often calls for oxidants like sodium hypochlorite, passing through controlled temperatures and pH to trigger the formation of the disulfide bridge between two MBT molecules. This step-by-step sequence builds up the two-ring structure in Di(Benzothiazol-2-Yl) Disulphide. Modern factories automate this sequence, measuring temperature, flow rates, and reactant levels in real-time to hit purity and batch-to-batch consistency. Release testing after crystallization and drying confirms that leftover reagents don’t stick around to contaminate the rubber-based products down the line.
Inside a curing rubber mix, Di(Benzothiazol-2-Yl) Disulphide breaks its own sulfur bridges, sharing those sulfur atoms with natural or synthetic rubber chains. This forms crosslinks—tiny bridges holding the rubber matrix together. More crosslinks mean better heat resistance, improved tensile strength, and less permanent deformation under stress. Some research outfits modify this chemical further, adding different side-chains or matching it with unique co-accelerators to fine-tune the curing for specialty applications, whether in extra-flexible shoe soles or rigid, oil-resistant seals. The chemistry allows for adaptability with process parameters while maintaining the backbone of strong sulfur links.
The chemical industry knows how to pile up alternate names: MBTS for short, more formally Dibenzothiazyl Disulfide or sometimes just “Disulfide accelerator.” Famous brands, such as Altax and Vulkacit, show up on procurement forms and invoices across continents. These synonyms help buyers and regulators cross-check materials, especially in global supply chains where naming conventions overlap. Suppliers sometimes build reputation on consistency and purity under these varied names, but the molecule inside the bag stays the same.
Safety officers in modern plants care about more than just gloves and masks. Handling Di(Benzothiazol-2-Yl) Disulphide brings responsibility: keep powder away from open flames, avoid inhalation of dust, and store it well away from acids or oxidizers. Skin and respiratory protection cut down exposure risks, especially during mixing or transfer. Regulatory bodies like OSHA and REACH set occupational exposure limits and demand thorough documentation for safety data sheets. Emergency response plans target spills and accidental releases, emphasizing the need for ventilation and containment. Checking safety protocols regularly and running practice drills helps everyone stay sharp in dealing with the compound gremlins that can crop up in an industrial environment.
Tire shops rely heavily on Di(Benzothiazol-2-Yl) Disulphide for truck and passenger vehicle tires. Conveyor belts in factories—moving coal, carrying food, or sorting packages—benefit from the enhanced wear properties and longer life granted by this accelerator. Seals and gaskets pressed into engines or pumps keep oil and steam locked tight, avoiding leaks that drive up maintenance costs and downtime. Footwear soles, sporting goods grips, and vibration-dampening mounts—every piece that counts on rubber’s blend of flexibility and resilience turns to this compound for reliability. Outside of pure rubber goods, it sometimes ends up stabilizing certain polymers or acting as a crosslinking agent in specialty plastics, giving manufacturers that extra edge for their next product revision.
University and industrial labs constantly search for improvements. Teams run controlled experiments swapping Di(Benzothiazol-2-Yl) Disulphide with alternative accelerators or pairing it with new co-agents. Their goal? Stronger, safer, greener rubber. Sustainability holds real weight in grant applications now, and these research programs have started tracking the chemical’s full life cycle—from raw-material extraction down to end-of-life breakdown in landfills or recycling streams. Papers detail the benefits of microencapsulation and surface-modified forms, hoping to cut back on chemical waste and increase efficiency in high-volume production. Real progress shines through in how finished products handle stress and environmental exposure, leading to lower rates of field failures.
Toxicologists and environmental scientists keep a close watch on compounds like Di(Benzothiazol-2-Yl) Disulphide. Lab tests on animals and short-term bioassays focus on skin irritation, respiratory effects, and long-term organ impact. Workers exposed to high dust concentrations risk respiratory irritation. Extended exposure at higher levels, based on some rodent studies, suggest a need for cautious handling. Disposal guidelines push for proper incineration and controlled landfill rather than open dumping, as trace residues could lead to slow soil or water contamination. Markets pay attention, in part from consumer movements demanding safer tires and child-safe toys, so the story of toxicity shapes future manufacturing and regulatory frameworks.
Looking forward, the pressure mounts to do more with less environmental impact. Sustainable tire ingredients and recyclable rubber blends drive demand for process chemicals to pull their weight without leaving a harmful legacy. Researchers hunt for faster-curing, lower-toxicity accelerators with the flexibility of Di(Benzothiazol-2-Yl) Disulphide but a lighter ecological footprint. Companies count on this foundation block, but innovation could bring new blends or hybrid catalysts, making both the pedal-to-the-metal world of racing tires and the everyday reliability of commuter gear safer and cleaner. It’s not just about keeping old machines running, but keeping pace with expectations on quality, price, and planet health. Real industry change often starts with a shift in the chemistry—no exaggeration to say that’s where opportunity lives and where the next breakthrough waits.
Walk into any tire shop or rubber factory, and the invisible supporting cast behind those tough, flexible materials includes a mouthful of chemicals. Among them, Di(Benzothiazol-2-Yl) Disulphide, sometimes known as MBTS or mercaptobenzothiazole disulfide, plays a key role. This compound shows up in rubber production far more than most people ever realize. During my early days working in a small mechanical workshop, I remember hearing older colleagues talk about all sorts of accelerators—MBTS always made the list. It always seemed a bit mysterious, but the story makes sense once you start looking at how rubber gets made.
Rubber isn’t tough or weatherproof straight out of the tree or petrochemical plant. The magic happens through vulcanization. Think of it as a controlled baking process, where heat, sulfur, and a handful of critical additives transform sticky latex into something that won’t melt in the sun or snap in the winter. Di(Benzothiazol-2-Yl) Disulphide steps in here as a vulcanization accelerator. Without this step, the process runs much slower and leaves behind weak, soft material. MBTS makes the sulfur cross-linking reaction move along briskly, producing tires and hoses with the strength needed for daily punishment.
MBTS finds regular work in everything from car tires to conveyor belts and shoe soles. The reason comes down to reliability and consistency—qualities that make a genuine impact for anyone who depends on rubber in harsh conditions. Taking the tire industry, for example, most producers favor MBTS because it ensures a steady cure rate and helps guard against premature aging of the final product. In daily life, these are the details that keep our boots from cracking and help car tires hold together mile after mile.
Like most industrial chemicals, handling MBTS calls for attention to safety. During its manufacturing or blending, proper protective equipment and smart ventilation protocols become crucial. Research points out that MBTS can cause skin or respiratory irritation if not managed responsibly. Some environmental concerns also arise, as benzothiazole derivatives can linger in the environment. The best plants and firms commit to gloves, fume extraction, and proper disposal systems, keeping workers and communities out of harm’s way.
The big question these days wraps around sustainability and alternatives. Watching the way green chemistry has grown, some in the rubber industry now search for accelerators with lighter environmental footprints. This isn’t just about following the latest trend—consumers and regulators alike push for products that last, stay safe, and tread lightly on the earth. Some companies put funds into research for new sulfur donors and safer boosters, hoping to keep production humming without the old pollution headaches.
As rubber use grows in cars, machinery, and daily goods, the call to innovate echoes louder. A practical step involves retraining staff and upgrading ventilation in factories that use MBTS. Direct investment in greener accelerator technologies is a bet many forward-thinking companies see as worthwhile. For buyers and end-users, looking for products certified by independent environmental bodies adds weight to customer demands. Everyone up and down the chain has a chance to reduce risks while keeping modern life rolling along.
Working in chemical labs has taught me that risks often hide where you least expect. Di(Benzothiazol-2-Yl) Disulphide, widely used as a rubber accelerator, brings certain hazards that deserve full respect. Just one careless move can lead to long-term health issues or environmental harm. Focusing on proven safety habits holds more value than trusting luck. Every time I see newcomers skip gloves because they’re just “quickly pouring,” I remember stories of skin rashes or breathing pain that followed.
Dust from rubber chemicals often lingers much longer than it appears. Skin irritation and respiratory problems rank high on the list of risks with Di(Benzothiazol-2-Yl) Disulphide, and inhaling even a small amount can lead to chronic cough or more serious reactions over time. Without a proper fume hood or local exhaust, dust becomes nearly impossible to avoid. I learned the hard way that a cheap dust mask doesn’t cut it: Only certified respirators block fine particles from reaching the lungs. Updating ventilation should always take top priority before loading out any sacks.
No matter how comfortable a workspace feels, direct contact with this compound spells trouble. Gloves, safety glasses, and lab coats form a basic shield. Nitrile or neoprene gloves hold up better than latex in my experience, and regular checks for pinholes prevent nasty surprises later. Goggles might feel excessive, but one splash into the eye can ruin more than a whole afternoon. Dedicated work clothes, laundered on site, cut down on accidental transfer to the car or home. After a decade in the field, I always go overboard with protective gear—because the headache of chemical burns or allergies never fades quickly.
Routine matters as much as emergency response. Every spill demands quick, focused action—damp towels for small quantities, vacuum systems for powders, never a dry broom that can whip up invisible clouds. Safe disposal rules keep accidents from spreading outside the workplace. Seal all waste in designated containers, label them clearly, and never let disposal routines slide for the sake of speed. Employees should know what to do, not just when supervisors watch, but at any moment. I’ve worked places where small lapses in clean-up turned into full-forced workplace shutdowns.
Training newcomers well and refreshing knowledge regularly gives everyone a better shot at avoiding accidents. Visual reminders—signs by chemical storage, checklists for PPE, emergency shower drills—make safety routines hard to ignore. Strong record-keeping tracks exposure incidents and reveals risks that aren’t obvious at first glance. When open conversation about near-misses happens, the whole team learns to spot problems before they get serious.Manufacturers can help, too, by improving packaging to cut open-bag spills and moving toward pelletized forms that release less dust. Companies who listen to frontline workers often spot risks missed by top-down rules.
Regulation can’t catch everything, but personal commitment always fills the gap. No shortcut pays off when dealing with chemicals that can stay with you for years. Direct action and teamwork build real safety—one habit, one reminder, one clean lab coat at a time.
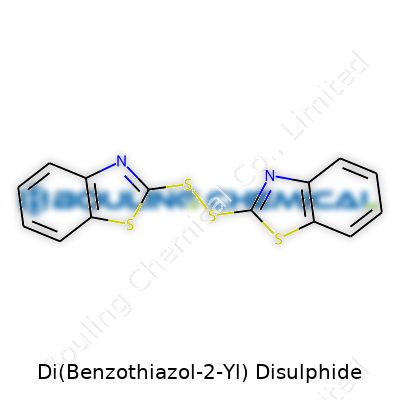
Scientists and engineers working with rubber or specialty chemicals know this compound by its formula—C14H8N2S4. The molecule’s structure builds on two benzothiazole rings connected through a disulfide bridge. Each benzothiazole features a bicyclic structure, where a benzene ring fuses with a thiazole. By linking two of them via –S–S–, the result is a chunky, stable molecule.
Structural diagrams show the sulfur atoms holding the rings together like a pair of firm, supportive hands. What stands out is the stability provided by the disulfide bond, giving this molecule a real backbone. The linkage can be represented as:
Benzothiazole-S–S-Benzothiazole
Where each "Benzothiazole" calls to mind a six-membered benzene ring fused to a five-membered thiazole. From a chemist’s perspective, the symmetry across the disulfide bridge brings both elegance and practical function.
Rubber doesn’t gain its strength and elasticity on its own. 2,2’-Dithiobis(benzothiazole), often called MBTS, acts directly in the vulcanization process—a chemical reaction that hardens rubber and changes its properties for use in countless everyday products. Tires, conveyor belts, gaskets—each owes much of its performance to molecules like MBTS.
The process works through sulfur cross-linking under heat and pressure. MBTS steps in to accelerate this reaction, getting those polysulfidic links to form more quickly and under better control. Think of early mornings in an auto workshop: that dependable, tough tire came about not by chance, but through research into which molecules foster just the right degree of toughness and flexibility.
Without such accelerators, vulcanization proceeds at a crawl, requiring more energy and time. MBTS not only speeds things up, it brings consistency and improves the lifespan of finished rubber goods. Tire blowouts and cracked hoses used to be far more common—better chemistry changed this.
Like any chemical with broad industrial use, MBTS raises important questions about safety and environmental impact. Direct handling can irritate the skin or eyes, and prolonged exposure during manufacturing or disposal could pose ecological risks. Years ago, I visited a rubber plant and saw the clear efforts made to minimize exposure: gloves, sealed systems, regular air monitoring. Regulatory agencies such as OSHA lay out permissible exposure limits. Workers receive training to respect these limits. Waste gets treated or incinerated to keep sulfur and nitrogen compounds out of water and soil.
Recycling or safer substitutes also draw interest. Chemists study ways to recover MBTS from old rubber or replace it with less hazardous accelerators. Progress often comes slower than we'd like, but improvements do show up—greener manufacturing and recycling help, even on a small scale.
The story of MBTS offers a lesson in balancing industrial progress with safety and sustainability. Factories adopt better dust controls, engineers tweak formulas for less residue, and labs keep exploring alternatives to reduce legacy chemical footprints. The path toward safer, more circular rubber production relies on careful design, solid regulations, and keeping an eye on long-term impact. By taking lessons learned from experience and data, industry can keep pressing for more responsible and forward-looking solutions.
Ask anyone who has worked around chemicals, whether in a classroom or on an industrial floor, bad storage habits invite big trouble. Di(Benzothiazol-2-Yl) Disulphide isn’t in every household, but rubber factories, research labs, and chemical warehouses use it. It carries a reputation for helping rubber products get their bounce, but nobody wants their workspace to bounce into a fire or release fumes from a simple storage mistake. My own years in a materials-handling environment taught me that careless storage starts a chain that ends in health scares, ruined investment, and headaches during inspections.
A little moisture turns powders like this one into sticky messes, which not only ruins product quality but can make it hard to weigh or add to formulas later. Some folks toss bags onto concrete floors, forgetting concrete sweats in humid months. Cardboard boxes don’t block moisture for long, so my old boss insisted on sealing containers tightly and storing them above the ground, on pallets or racks. Laboratories swear by using thick, air-tight drums with sturdy lids for a reason. Once water finds a tiny gap, you can expect lumps and sometimes minor chemical reactions.
Most common stories of accidents I’ve seen start with a pile of containers too close to the heating system or left under a skylight. This chemical stands up well in stable conditions but reacts under strong heat, breaking down or releasing sulfur smells that stay stuck in a warehouse for hours. Ideally, stash it in a spot away from direct sun and heating equipment. Some smaller shops put up simple shade cloths over their shelves. Heat barely gets discussed during storage training, but it makes the difference between safe product and staff complaints or dangerous leaks.
Strong ventilation changes the entire game. I remember a facility without windows in the storage area. Even without disasters, the stale smell lingered and made people avoid that wing. Good airflow keeps any unexpected vapors from collecting. Simple exhaust fans, open windows, or specially-designed vents help reduce inhalation risk and control odors. There’s value in routine walk-throughs to check for leaks, cracked containers, or crust forming on the lids – a solid habit that can prevent expensive inventories from going bad.
Mixing up containers or stacking strong oxidizers right next to this one ends in emergency drills. Team members learned to color-code shelves and bins. Segregated chemical racks with clear labeling help avoid confusion, especially where several powders and liquids share space. Over the years, I’ve seen burn marks from accidental spills that reminded everyone why separation matters. Simple written rules and a storage map kept things straightforward.
Good gloves, safety goggles, and long sleeves become as important as proper containers. Training staff to handle spills or forgotten open containers keeps warehouses safer. Every refresher course I attended hammered home not to touch the face or eyes after using these materials. Real-time spill kits – gloves, absorbent material, clearly marked bins – belong close by, not buried in an office across the building.
I’ve learned that prevention, not fancy technology, keeps costs down and people healthy. Basic, regular checks and respect for storage recommendations make a bigger difference than most realize. Di(Benzothiazol-2-Yl) Disulphide rewards good habits; safe storage means the difference between a smooth production schedule and a week ruined by cleanup and lost materials.
Many in the rubber manufacturing world know this chemical by its common abbreviation, MBTS. Used as an accelerator in making tires, belts, and hoses, it ends up woven into the daily lives of factory workers and their communities. My own visits to small factories have given me a real sense for how easily powders like MBTS can scatter. Fine dust clings to hands, workbenches, and even noses of those who spend all day around the material. The presence lingers, often unnoticed.
Most workers dealing with MBTS wear gloves, but I’ve seen forgotten goggles or neglected masks more times than I can count. Skin contact or inhalation brings real trouble. Chronic exposure can trigger allergies, asthma-like symptoms, and skin irritation. Research published in the International Journal of Occupational Medicine shows signs of respiratory distress among longstanding rubber workers. Eyes sting, breaths grow short—these are not stories pulled from statistics, but from lived experience in hot, noisy workshops.
Moving outside the factory, MBTS can make its way into streams and soil. Not far from a tire plant I visited last year, a creek ran cloudy after a rainstorm. Testing revealed higher levels of benzothiazole derivatives downstream—a clue that runoff carries more than just dirt.
Studies out of Europe and East Asia have pointed out that MBTS doesn't easily break down in nature. Once in rivers or lakes, it persists and affects fish in subtle but worrying ways. Behavioral changes, lowered reproduction, and even bioaccumulation show up in the scientific literature. Researchers in China tracked declines in aquatic insect populations below chemical plants, tracing the source to tire additives like MBTS. What surprises many people is how little dilution by a big river can lessen the pollution, especially when inputs continue year after year.
Workers bring traces of the chemical home on their clothing and shoes. As I’ve learned from union health advocates, children can pick up residues from laundry baskets or carpets. Over time, even low-level exposures begin to add up. Chronic low-dose chemical exposure rarely prompts sirens or big headlines, but it can sink its teeth into communities with problems that health clinics are slow to diagnose.
Several programs offer hope. One steel-belted rubber operation I visited switched to enclosed mixing equipment, sharply dropping dust levels. Community monitoring and annual health checks catch early signs of chemical-related illness. Germany and Japan have both set stricter workplace limits and invested in water treatment plants near major rubber hubs.
Safer handling practices, real-time air monitoring, and proper worker training matter just as much as any regulatory limit. Some companies have tried alternative chemicals that break down faster in nature, but the quest for safer substitutes takes more research and investment from the big players.
Communities near factories benefit when local government keeps a close watch on air and water. Independent testing, reported in plain language, helps workers and residents understand the stakes.
Personal stories—someone’s irritated skin, a lost fish run—remind us that the health and environmental hazards of MBTS don’t stay abstract. Factories, workers, neighborhoods, and natural streams thread together into one story. Action doesn’t come easy, but it starts with practical safeguards, investment in substitutes, and the courage to ask what price we pay for a smoother ride on rubber wheels. Facts on the ground, real voices, and clear-eyed monitoring point toward solutions everyone can live with.

| Names | |
| Preferred IUPAC name | bis(1,3-benzothiazol-2-yl) disulfide |
| Other names |
MBTS Benzothiazyl Disulfide MBT Disulfide Vulcanization Accelerator MBTS Bis(benzothiazol-2-yl) disulfide |
| Pronunciation | /daɪˌbɛnzoʊˌθaɪəˈzoʊl tuː aɪl daɪˈsʌlfaɪd/ |
| Identifiers | |
| CAS Number | 120-78-5 |
| Beilstein Reference | 1208731 |
| ChEBI | CHEBI:34989 |
| ChEMBL | CHEMBL191833 |
| ChemSpider | 173405 |
| DrugBank | DB11274 |
| ECHA InfoCard | 06f6b10e-ea43-4537-bd9e-684e7e17954e |
| EC Number | 233-067-2 |
| Gmelin Reference | 236715 |
| KEGG | C19596 |
| MeSH | D003971 |
| PubChem CID | 8701 |
| RTECS number | GU7850000 |
| UNII | NKW87N0GY7 |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C14H8N2S4 |
| Molar mass | 332.5 g/mol |
| Appearance | Light yellow powder |
| Odor | Faint characteristic odor |
| Density | 1.42 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.86 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 1.62 |
| Basicity (pKb) | 13.2 |
| Magnetic susceptibility (χ) | -33.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.732 |
| Viscosity | Viscosity: 15 mPa·s (20°C) |
| Dipole moment | 2.12 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 576.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -43.7 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -875 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | D11AX18 |
| Hazards | |
| Main hazards | May cause cancer. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. Toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H317, H410 |
| Precautionary statements | P261, P273, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | > 221°C |
| Autoignition temperature | Autoignition temperature: 593°C |
| Lethal dose or concentration | LD50 oral rat > 5000 mg/kg |
| LD50 (median dose) | > Oral rat LD50: 5000 mg/kg |
| NIOSH | PY5600000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Di(Benzothiazol-2-Yl) Disulphide: "5 mg/m3 |
| REL (Recommended) | 30 mg/m3 |
| Related compounds | |
| Related compounds |
Mercaptobenzothiazole 2-Mercaptobenzothiazole zinc salt Tetramethylthiuram disulfide Dibutylthiourea Diphenylguanidine |