Researchers first synthesized di-2-thienylglycolic acid methyl ester in a wave of discovery focused on sulfur-containing heterocycles. In the sixties and seventies, chemists worked with thienyl compounds for their electronic properties and their potential as building blocks in pharmaceuticals. Academic groups used basic esterification routes, modifying carboxylic acids with alcohols, but the thienyl rings set this compound apart, opening up fresh ground in research. The world of organic synthesis got a nudge from developments around this molecule since its unique scaffold supported subsequent work in drug, dye, and conductive polymer design.
Di-2-thienylglycolic acid methyl ester, often sold as a yellow twinkling solid or viscous oil, features two thiophene rings—these bring in a sturdy backbone and electron-rich character. Chemists value this ester for its ability to slot into synthetic sequences as a flexible intermediate. In industry, suppliers market it to R&D centers focusing on medicinal chemistry or electronics because it plays well with advanced coupling reactions and modifications at the ester or thienyl sites.
The compound’s molecular formula reads C11H10O2S2, and its molar mass hovers around 238.3 g/mol. Its melting point stays loose, so it’s usually managed as an oil, with solubility faring well in common organics like dichloromethane or ethyl acetate. The electron-rich thienyl rings produce a faint, mercaptan scent, reminding you it comes from the sulfur world. Lab notes often warn about light-sensitivity, and slight shifts in storage temperature can affect shelf life. UV and NMR spectroscopies display distinctive thienyl peaks and ester carbonyl signatures, providing good markers for purity checks.
Industrial suppliers define content purity at the 97% or above mark according to gas chromatography and proton NMR analysis. Labels note “For Research Use Only” because regulatory agencies haven’t cleared it for food or drug use. Bottles feature lot numbers for traceability, and chemical safety information echoes throughout the documentation. Product sheets provide batch-specific melting points, color observations, and water levels, since minor impurities can shift reaction outcomes downstream in synthesis.
Standard preparation starts with di-2-thienylglycolic acid, itself derived by cross-coupling methods or Friedel–Crafts-type acylation. The methylation follows a textbook Fischer esterification: reflux with methanol and a dash of acid catalyst like sulfuric acid, washing with brine, and drying over magnesium sulfate. Experienced chemists stress the need for anhydrous conditions, since water in the system tankers with both yield and color. Rotary evaporation helps recover the finished ester. Some labs have experimented with milder, greener methods—using solid acid or enzymatic catalysis—but these remain niche in commercial production.
Both the ester and thienyl rings allow for fine-tuning. Basic hydrolysis gives back the free acid, essential for bioconjugation work. Chemists can react the methyl ester with alkali to yield the sodium or potassium salt for water solubility. The thienyl rings, being electron-rich, go into halogenation, nitration, or Suzuki couplings, making the molecule a good anchor for advanced materials or pharmaceutical design. Some teams use it as a precursor to cross-linked polymers, taking advantage of the thiophene segments for conductivity.
In catalogues and journal articles, you’ll see the compound called methyl di(2-thienyl)glycolate or methyl 2,2′-bithienyl-2-yl-glycolate. Vendors list it alongside thienyl derivatives, grouping it into a family essential for electronic and pharmaceutical exploration. The variety of names sometimes complicates literature review, so CAS numbers and structural diagrams clear up any confusion.
Handling di-2-thienylglycolic acid methyl ester asks for thoughtful care. Spills on skin sting, and its slight volatility brings risks if left open on the bench. Lab protocol involves nitrile gloves and goggles. Fume hoods keep fumes from accumulating, especially during scale-up syntheses. Waste products get channeled to organic halide or sulfurous solvent streams for special disposal. Labels include health warnings about eye and respiratory irritation and restrict direct contact. The compound’s low flammability makes physical hazards less of an issue, but material safety data sheets always stay close at hand during use.
Medicinal chemists put this ester to use in early-stage drug screening—mainly as a scaffold for anticancer, antifungal, or CNS-active compounds. Organic electronics researchers look for stable, sulfur-containing molecules, and thienyl esters like this one support charge transfer, so they feature in preliminary designs for organic solar cells and field-effect transistors. Dyes and pigments with thienyl units get brighter colors and better durability. The molecule’s adaptability opens doors for polymer chemistry researchers, especially those pushing for more environmentally friendly plastics.
University and private labs keep working with di-2-thienylglycolic acid methyl ester, aiming to push its potential further into pharmaceutical and material markets. Current work centers on modifying the ester to introduce new active sites, hoping to lock in disease-fighting activity or charge-transfer properties. Polymer chemists experiment with new cross-linking protocols in search of stronger, more conductive materials based on the thienyl core. Funding agencies have shown support because the sulfur and oxygen mix delivers fresh opportunities in medicinal and materials chemistry. Publications keep rising, reflecting a broadening interest in taking small heterocycles and wiring them into new technologies.
Animal and in vitro models have examined acute and chronic toxicity for thienyl esters. High doses bring out hepatorenal effects, as organosulfur compounds often stress the liver and kidneys. Methyl esters, absorbed quickly, linger in fatty tissues, so studies note bioaccumulative potential. Toxicologists call for more data on metabolites and environmental breakdown products. Lab safety guidelines recommend minimizing exposure, keeping use to technical hands, and not releasing lab-scale waste into regular drains due to the risk of aquatic toxicity. In my experience, the protocol discipline around this chemical matches what I’ve seen with more harshly regulated synthetic intermediates—a nod to its power and risk.
Di-2-thienylglycolic acid methyl ester stands at a turning point, with more eyes on sulfur-rich heterocycles at the crossover between medicinal and materials chemistry. Researchers see the value in its unique combination of stability and modifiability, pressing ahead with new approaches in organic synthesis, catalysis, and electronic materials. Improvements in greener preparation and breakdown routes might ease concerns about toxicity and waste, helping bring more sustainable methods into labs and production plants. More advanced drug discovery platforms, powered by AI-driven molecular screening, bring hope for new uses, while the data emerging from ongoing studies feeds back into an expanding toolkit for future innovation.
At a glance, the name “Di-2-Thienylglycolic Acid Methyl Ester” sounds like something better left to those in white lab coats. Dig into the components, though, and a clear structure starts to reveal itself. The backbone features a glycolic acid, modified with two thienyl groups and finished off with a methyl ester. This isn’t just textbook chemistry; these building blocks hint at the compound’s potential applications in research and industry.
Think of glycolic acid: two carbons, a carboxylic acid, and a hydroxyl group. Here, both hydrogens on the alpha carbon have been swapped for thienyl rings, which are five-membered sulfur-containing aromatic systems. These rings lend both stability and rich chemical reactivity. Now, put a methyl ester in place of the free acid—this swap trades hydrogen for a methyl group, raising solubility in organic solvents and reducing the acidic bite of the core molecule.
Pulling this together for the chemistry enthusiasts, the structure follows this framework: You have the central carbon attached to a carboxylate methyl ester (COOCH3), and two thienyl groups directly linked to the same central carbon atom. The simplified chemical formula lands as C11H10O2S2. Visualize it, and there’s a neat symmetry: two large rings flanking a short carbon chain, all tied together with an ester finish.
Chemists tend to scan a structure for activity hotspots. Those thienyl rings, acting as aromatic electron clouds, open doors to further reactions or interactions with biological molecules. Having an ester group on the end invites hydrolysis—break it down, and you release the acid back. These tweaks matter, especially in fields like medicinal chemistry or materials science. The presence of aromatic sulfur offers unique electronic properties, attracting attention in studies on molecular electronics or ligands for metal complexes.
Safety and HandlingSpeaking from lab experience, working with di-thienylglycolic esters isn’t casual. Standard precautions—gloves, goggles, solid ventilation—aren’t optional. The aromatic sulfur compounds can have sharp odors or skin-irritating traits, and the methyl ester doesn’t improve eatability. Proper storage and waste disposal keep risks at bay and protect researchers and the environment.
Research keeps pushing boundaries, and a compound with thienyl rings tacked onto a methyl ester sparks curiosity among organic chemists. With careful design, new derivatives or analogues might pop up in search of better catalysts, drug candidates, or electronic materials. The right collaboration between synthetic chemists and analysts leads to important discoveries, but the work begins with a strong understanding of how all those bonds and atoms fit together.
In a world that depends on reliable facts and hands-on experience, paying attention to both the chemical structure and the practical steps in the lab makes all the difference. Knowledge doesn’t just sit in a textbook or a database; it grows each time someone runs a reaction, measures a yield, and thinks creatively about what to try next.
Chemists and product developers keep looking for molecules that can push technology forward, and Di-2-Thienylglycolic Acid Methyl Ester belongs to that club. Anyone working in fine chemicals or advanced materials might have run across this compound, especially if they spend much time in organic synthesis or specialty manufacturing. Years ago, I spent days in the lab working on heterocyclic chemistry, and these sulfur-rich compounds always seemed to pop up when pushing for new reactions in medicinal or materials science.
One of the main uses for Di-2-Thienylglycolic Acid Methyl Ester sits in the world of organic synthesis. People use it as a building block when constructing more complex sulfur-containing molecules. Its thienyl rings and ester group bring unique reactivity. For research labs, it often stands in as a precursor while developing new drug molecules or agrochemicals. This flexibility in synthesis means chemists can easily transform it into acids, amides, or even thioethers, depending on the project.
Industries focused on electronics or advanced polymers rely on specialty intermediates. Years ago, while collaborating on organic semiconductor research, I saw thienyl-based esters slide into early-stage tests for conductive polymers. The electron-rich structure of Di-2-Thienylglycolic Acid Methyl Ester supports charge transfer, an essential property for improving conductivity in organic light-emitting diodes and next-generation solar cells. Performance gains might seem incremental, but pushing those device boundaries often starts with molecules like this one.
Although the compound itself doesn't show up in medicine cabinets, chemists use it as a key step on the way to more valuable targets. Its framework suits the construction of diverse pharmacophores, especially in research hunting for new anti-inflammatory or anticancer agents. The synthetic flexibility provides an easy route into thienyl derivatives, which pop up in a surprising range of preclinical studies. Experience reminds me that even minor changes in such base chemicals can have a profound impact on biological activity downstream.
Beyond high-tech fields, Di-2-Thienylglycolic Acid Methyl Ester finds occasional use in the flavor and fragrance sector. The thienyl structure brings sulfurous, earthy notes, which certain blend designers crave for specialty product launches. Dye chemists also leverage its aromatic backbone while searching for pigments carrying distinctive electronic properties.
Every time a new molecule gets put to work, safety and environmental impact come up for discussion. The industry’s history with thienyl compounds includes both breakthrough products and some problematic legacies, so safe handling matters. Current chemical guidelines stress responsible disposal and minimal exposure. Plenty of emphasis now rests on greener synthesis and investigating longer-term impact, in part driven by tighter global controls and a rising demand for sustainable chemistry.
Better supply chains and collaborations between research institutions and industry can help unlock new uses for Di-2-Thienylglycolic Acid Methyl Ester. Synthetic chemists already work on more efficient routes to cut waste and energy use. Government and industry oversight brings needed scrutiny to maintain trust and bolster safety. By bringing together experience from the lab bench and lessons from industry, the field still has room to improve both the performance and the footprint of this versatile chemical.
Anybody working with chemicals or pharmaceuticals knows the worth of accuracy, especially on the molecular level. Di-2-Thienylglycolic Acid Methyl Ester is not a household name, but it finds its way into specialty chemical research, often serving as an intermediate for more advanced compounds. Sitting at the intersection of organic synthesis and analytical chemistry, this molecule’s molecular weight — 266.34 grams per mole — holds the key to handling it properly.
Back in my early lab days, I miscalculated the required molecular weight for a simple ester. The experiment went off track, and I learned fast. For compounds like Di-2-Thienylglycolic Acid Methyl Ester, there's no luxury for slip-ups. Measuring reagents by weight gets precise reactions; dusty or casual measurements don’t cut it. This molecular weight allows chemists to figure out how many grams are necessary for a certain number of moles — a direct impact on yield and purity. Every time someone synthesizes a batch without this number, the process turns messy, often with wasted time and resources.
Think of a typical esterification or coupling reaction. These often depend on exact stoichiometry — miss by a fraction, and you won’t hit the mark. Di-2-Thienylglycolic Acid Methyl Ester’s molecular weight of 266.34 bridges the instructions in the textbook and the beaker on the bench. Some academic labs keep cheat-sheets of common molecular weights, so no one gets tripped up in a calculation at the wrong moment. For industry, scaling up without a precise number means batch failures and lost revenue.
Pharmaceutical and specialty chemical companies don’t play games with unknowns. Products developed using Di-2-Thienylglycolic Acid Methyl Ester face rounds of testing for consistency and potency — the molecular weight provides the foundation for these quality checks. Analytical methods, such as High-Performance Liquid Chromatography (HPLC), use this value to detect and quantify the ester in a production sample. Miss the mark and you can’t trust what you measured, which can ripple out into full product recalls or worse.
Lab accidents linked to faulty measurements often arise from using the wrong molecular weight. During my mentorship years, I saw chemists scribble conversions in the margins of their lab notebooks, double-checking each step to prevent a chain reaction of costly errors. Today, digital databases and integrated laboratory management systems help chemists keep track of these details, but understanding the importance behind that 266.34 grams per mole stays critical. Rushing leads to slip-ups; new researchers benefit from guided checklists, regular protocol reviews, and access to up-to-date chemical properties.
Open access databases like PubChem and Reaxys equip researchers with up-to-date values, while strong lab training reduces mistakes. Clear labeling and digital batch records streamline tasks for busy teams. Knowledge-sharing between experienced chemists and new hires spreads best practices. Professional certifications and external audits support labs that handle complex molecules, ensuring consistent accuracy across projects.
Precise molecular weight isn’t just a number; it’s a cornerstone for scientific reliability and commercial trust. Chemists, whether fresh from academia or seasoned in industrial roles, depend on accurate data, documented procedures, and a respect for the building blocks of every compound handled. Di-2-Thienylglycolic Acid Methyl Ester, with its unique structure and molecular weight, serves as a daily reminder that none of these details is too small to matter.
I’ve worked around lab benches and chemical stockrooms for over a decade, and I’ve learned that storing organic esters, especially ones with sulfur in their structure like Di-2-Thienylglycolic Acid Methyl Ester, isn’t just a boring paperwork exercise. The cost of sloppy storage runs high—think not just about ruined reagents, but health hazards, insurance headaches, and time wasted over cleanup emergencies. This ester often draws nervous glances from lab managers, and for good reason: volatile organics tend to cause trouble if left unchecked, especially during those times when everyone’s attention slips.
Heat can do strange things to chemicals—sometimes reactions that textbooks barely mention. For this ester, warm storage conditions aren’t just a way to shorten its lifespan; even in sealed bottles, decomposition releases fumes. My nose has gotten a whiff once or twice—never pleasant.
Best bet? Look for a chemical refrigerator or a cool storage room. Most reputable suppliers ship this compound with advice to keep it at eight degrees Celsius or colder. I’ve watched bottles break down after a weekend in a sunny window. Moisture poses a hidden danger, too. Water in the air doesn’t just spoil chemicals through hydrolysis; in the worst-case scenes, it can corrode caps, stick glassware shut, or set off the slow development of nasty byproducts.
Years ago, I talked to a chemist who had tossed all his bottles in boxes, confident he’d labeled them well. One day, he pulled out his methyl esters, only to find sticky residue clinging to the threads. The problem wasn’t the labels—it was air creeping in, and a loose cap. For Di-2-Thienylglycolic Acid Methyl Ester, your container should be airtight, chemical-resistant (glass with good PTFE-lined caps works well), and fully closed between uses.
Glass is king, especially for reactive organics. Avoid plastic containers unless they’re specifically rated for organic acids and solvents. I've seen low-quality bottles leach, and the ester turned cloudy in days.
Too many forget legal requirements. In many countries, local law dictates how you handle and store hazardous organic compounds. These laws don’t exist just for compliance—they grew out of real accidents. Storage rooms need proper ventilation. Labs should never shrug off the value of chemical safety cabinets with correct signage and spill trays. Mixing sulfur-containing organics near acids or strong oxidizers? That’s just looking for an exothermic reaction.
Material Safety Data Sheets (MSDS) get ignored, yet they map out the worst-case scenarios and push for personal protective equipment (PPE) even during routine transfers. I’ve found the investments in safe storage cut down on staff injuries and batch losses remarkably.
Clearing up space and reorganizing shelves rarely seems fun in the moment, but regular lab audits protect both staff and investment in expensive chemicals. Inspections don’t need to be complicated. A good storage setup puts Di-2-Thienylglycolic Acid Methyl Ester on a lower, stable shelf (to minimize fall risk), inside a tray to catch leaks, far from direct sunlight or heat ducts. Safety training pays off most the day someone drops a bottle, and every bit of prep counts.
Anyone working around Di-2-Thienylglycolic Acid Methyl Ester ought to know the symptoms of inhalation or skin contact and make sure emergency eyewash and shower stations are close at hand. It’s not just about obeying a checklist; it’s about making sure the compound serves its purpose without turning into the biggest problem in the room.
Working with chemicals in a lab or manufacturing environment always carries certain risks, and Di-2-Thienylglycolic Acid Methyl Ester is no exception. This type of compound features two thienyl rings, which can introduce some unique hazards—everything from inhalation risks to problems with skin contact or accidental ingestion. With hazardous chemicals, a moment of carelessness can have long-term health effects. A lot of exposure risks don’t really show up until much later, so it's easy to underestimate the danger at first.
I once helped train a new chemist who believed a fume hood could handle anything. Over time, he let his guard down, stopped changing gloves between tasks, and got a nasty skin rash. The lesson? PPE is not just a checklist—it’s a barrier that stands between your health and the unknowns that each bottle and beaker can hide.
Nitrile gloves, a lab coat with cuffs, and safety goggles come standard for a reason. Splash protection matters, especially with organosulfur compounds that can irritate skin, eyes, or airways. Wearing a fitted respirator also makes sense if you’re transferring the compound in larger amounts or encountering dust or aerosol. Closed shoes and chemical-resistant aprons can cut down the chances of accidental exposure.
Even if a chemical has no noticeable smell, invisible vapors can still do damage. For this compound, always handle it inside a working fumehood. Don’t leave open containers on benches. Airborne particles can hang around, especially if ventilation gets weak. I’ve seen air sensors spike just by unsealing certain chemical bottles out in the open.
After work is done, proper storage stops small issues from becoming emergencies. Di-2-Thienylglycolic Acid Methyl Ester belongs in a tightly sealed container, kept cool and out of direct light. Flammable cabinets and desiccators aren’t just lab furniture—they play a big role in keeping things stable. Label everything with clear hazard signs, date of receipt, and your initials. This makes it easier for others to identify the chemical and know if it should be disposed of or returned to stock.
Even with good preparation, the unexpected still happens. I’ve dealt with more than one minor spill in my career. In each case, absorbent pads, disposable towels, and chemical waste bags kept the spread to a minimum. After cleaning up, washing hands with soap and plenty of water plays a big role in blocking any lingering harm. Each lab should have a spill kit in plain sight, and every member of the team should know how to use it before an accident hits.
OSHA and local safety agencies publish guidelines for handling organosulfur compounds. Reviewing Safety Data Sheets (SDS) before starting any work comes standard in responsible labs. Annual refresher training might feel redundant, but each session gives reminders that can stop shortcuts before they start. Good habits build up over time. I always encourage colleagues to speak up about any uncertainty—silence is never worth the risk.
At the end of the day, safety in the lab works best as a shared goal. No single procedure or checklist compares with the culture of double-checking, quick reporting, and open communication. Whether you’re new to the field or a seasoned chemist, looking out for your own health and those around you keeps the work productive, the lab open, and everyone heading home in good shape.
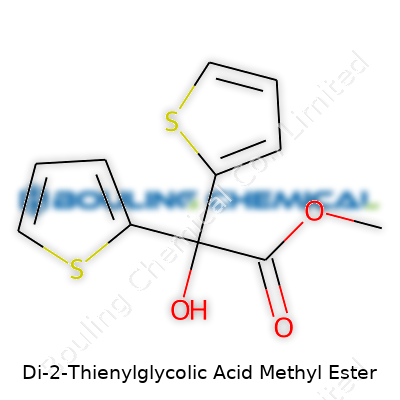
| Names | |
| Preferred IUPAC name | Methyl 2,2'-bithien-2-yl-2-oxoacetate |
| Other names |
Methyl 2-(2-thienyl)glyoxylate Methyl 2-thienylglyoxylate Methyl di-2-thienylglycolate Methyl 2-thiopheneglyoxylate |
| Pronunciation | /daɪ-tuː-θaɪˈɛnl-ɡlaɪˈkəʊlɪk ˈæsɪd ˈmiːθəl ˈɛstər/ |
| Identifiers | |
| CAS Number | 6786-77-6 |
| 3D model (JSmol) | `3D model (JSmol) string` for **Di-2-Thienylglycolic Acid Methyl Ester** (Methyl 2,2-di(thiophen-2-yl)acetate): ``` CC(=O)OC(C1=CC=CS1)C2=CC=CS2 ``` *(This is the canonical SMILES string typically used for JSmol 3D modeling)* |
| Beilstein Reference | 1596968 |
| ChEBI | CHEBI:91282 |
| ChEMBL | CHEMBL1507873 |
| ChemSpider | 3069246 |
| DrugBank | DB08240 |
| ECHA InfoCard | 100.051.634 |
| EC Number | 68784-19-8 |
| Gmelin Reference | 607298 |
| KEGG | C18920 |
| MeSH | D004088 |
| PubChem CID | 25279883 |
| RTECS number | XN9275000 |
| UNII | M63WVF7H1M |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | DTXSID5070897 |
| Properties | |
| Chemical formula | C9H8O2S2 |
| Molar mass | 256.31 g/mol |
| Appearance | White to light yellow solid |
| Density | 1.3 g/cm3 |
| Solubility in water | Insoluble in water |
| log P | 1.96 |
| Acidity (pKa) | 4.21 |
| Basicity (pKb) | 6.02 |
| Magnetic susceptibility (χ) | -63.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5700 |
| Viscosity | Viscous liquid |
| Dipole moment | 4.07 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 476.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | No data |
| Std enthalpy of combustion (ΔcH⦵298) | −3941 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | N02BG06 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | Warning. Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 86.4 °C |
| Lethal dose or concentration | LD50 oral rat 312 mg/kg |
| LD50 (median dose) | LD50 (median dose) = 1600 mg/kg (rat, oral) |
| NIOSH | MR1600000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 0.2 mg/m³ |
| Related compounds | |
| Related compounds |
Methyl Glycolate Thiophene-2-carboxylic acid Di-2-thienylacetic acid Ethyl di-2-thienylglycolate Di-2-thienylketone |