Organic chemistry wouldn’t be the discipline we know now without pioneering work on chiral amino acids. D-(+)-Proline has a place in this long story, which stretches back well before the structure of proteins was solved. In the late 1800s, scientists started unraveling the confusing web of optical isomerism. Emil Fischer, a titan in this field, recognized amino acids as the bricks behind life’s architecture. He helped clarify why one mirror-image form—like D-(+)-Proline—might turn up in select microbes or plants, even though nature mostly favors the L-form in animal proteins. D-(+)-Proline won’t leap out from a list of essential amino acids, but it pops up just enough in biochemistry labs and synthetic shops to keep chemists on their toes. Over time, labs found more reliable ways to separate D- and L- enantiomers. Better optical resolution and fermentation tech kept this compound relevant for researchers peering into stereochemistry, the way enzymes work, and eventually asymmetric synthesis, which exploded in the late twentieth century.
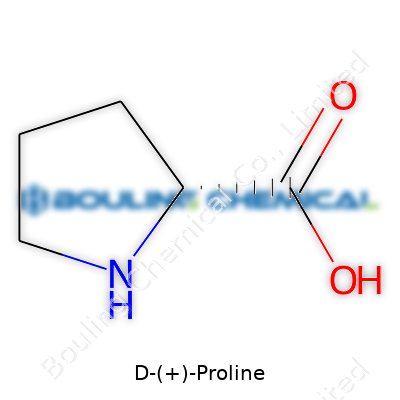
D-(+)-Proline is a cyclic, secondary amino acid. In everyday laboratory circles, chemists call it a building block—a starting point for constructing more complex molecules. Unlike regular straight-chain amino acids, proline’s side chain loops around to meet the nitrogen, giving the molecule a rigid ring structure. The D-(+)-form refers to the right-handed isomer, rare in animal proteins but useful for steering chemical reactions in a particular direction. D-(+)-Proline comes off-white or colorless, typically in crystalline or powder form, easy to handle yet packed with potential, especially for synthetic chemists looking to control the direction and outcome of their reactions.
Physical chemistry tells us D-(+)-Proline hits its melting point around 220°C (with decomposition). It dissolves well in water, a trait that matters whether mixing it for chemical reactions or conducting biological assays. The molecule carries the formula C5H9NO2 and weighs in with a molar mass just under 115 g/mol. As a zwitterion at neutral pH, it holds both a positive and a negative charge, which helps it sit comfortably in water-based systems. Its optical rotation is positive, confirming its right-handed (dextrorotatory) nature. It doesn’t flash, fizz, or reek—crucial in daily laboratory work, where predictability counts.
Every legitimate supplier provides a detailed certificate of analysis for D-(+)-Proline, noting purity—often 98% or higher for synthesis work—plus moisture content, melting point, and optical rotation. Labels require hazard information, batch numbers for traceability, and the CAS number 344-25-2. Analytical standards demand regular checks by techniques like NMR, IR, and HPLC, locking in trust for pharmaceutical, biotechnical, and food projects where regulatory compliance holds weight. Operations typically follow ICH guidelines, keeping contaminants such as heavy metals, residual solvents, and microbial counts below tough thresholds. Each batch needs proper storage instructions, given the risks from moisture and cross-contamination in multi-use facilities.
Most industrial-scale D-(+)-Proline isn’t harvested from nature. Instead, it comes from chemical or enzymatic synthesis. One classic method involves racemization—converting all forms to a mixture—then using enzymatic techniques to isolate the D-form. Fermentation through specific bacterial cultures can yield D-(+)-Proline directly, benefiting green chemistry movements by reducing the need for harsh chemicals. Chemical synthesis usually starts with pyrrolidine derivatives and involves steps like alkylation, hydrolysis, and crystallization. Each step requires tight control to avoid creating unwanted byproducts or failing to separate the target enantiomer. Increasingly, companies push biocatalysis to the front, looking for lower waste and easier scaling without heavy metals or solvents.
D-(+)-Proline anchors some iconic reactions in organic synthesis. One famous use is in asymmetric catalysis—manipulating it as an organocatalyst to trigger reactions like the aldol condensation, which makes complex molecules in a single step. Reactive centers—carboxyl and amine groups—open doors for attaching D-(+)-Proline to other molecules. Protecting groups (such as Boc or Fmoc) allow chemists to mask sections of the molecule and carry out multi-step syntheses for pharmaceuticals or peptide research. D-(+)-Proline stands up well to a host of transformations. Oxidation, acylation, halogenation, and peptide bond formation rank high among its chemical adventures. Research often tweaks the ring—substituting at position 4—to create N-substituted or fluorinated proline analogs, chasing new biological or catalytic properties.
D-(+)-Proline appears in catalogs with names like (R)-Proline, D-Pro, or 2-pyrrolidinecarboxylic acid (D-form). Suppliers may use EINECS number 206-481-4. Some research papers refer to it as dextrorotatory proline. The different names all point back to the same cyclic amino acid, but clarity in labeling matters in regulatory and R&D settings—mistaking D- for L- can derail syntheses or invalidate biological studies. This is not an interchangeable term; getting the enantiomer right sits at the core of its value.
D-(+)-Proline earns a reputation as a low-hazard material under regular lab handling. Standard chemical hygiene—gloves, goggles, lab coats—provides basic protection. Its dust can diminish air quality if carelessly poured or weighed, so fume hoods make sense on larger scales. Bulk users follow local and federal chemical regulations, usually guided by GHS and REACH labeling. Spills should be swept, and excess amounts kept away from drains. Waste typically moves through standard chemical disposal channels, avoiding unnecessary environmental exposure. Any scale-up process, especially when modifying proline or making derivatives, demands ventilation and spill containment—worse with chlorinating agents or reactive solvents. Regular risk assessments and staff training reinforce safe practices, particularly in facilities serving pharmaceutical production or university research.
D-(+)-Proline works quietly behind the scenes in many research labs. Its organocatalytic ability stands out; in the last two decades, scientists discovered how it can prompt carbon–carbon bond formation without metal catalysts. This offers cleaner, more sustainable synthesis routes for pharmaceuticals and fine chemicals. D-(+)-Proline steps into peptide synthesis, making D-amino acid peptides or mixed enantiomer chains. Some specialty pharmaceuticals require D-versions to improve metabolic stability or block unwanted enzymes. In analytical chemistry, D-(+)-Proline can help resolve racemic mixtures or act as a standard for instrument calibration. Agri-chemical research explores proline’s analogs as plant growth regulators, though the L-form usually takes center stage. Food chemistry sometimes uses D-(+)-Proline to study protein hydrolysis or digestion simulations in non-animal systems. It also piques interest in material science as a chiral modifier for liquid crystals or select metal-organic frameworks.
In academic and industrial labs, D-(+)-Proline brings an edge to reaction development. Its robust use in organocatalysis opened doors for greener methods, cutting down on metal catalyst waste and cost. Researchers push boundaries, examining ways to improve selectivity, activity, and general applicability of D-(+)-Proline in new types of reactions—Michael additions, Mannich reactions, and even some pericyclic transformations. Chemists modify proline’s skeleton to invent next-generation catalysts, seeking greater substrate range or milder reaction conditions. Work continues on enzymatic kinetic resolution, hoping to scale up D-(+)-Proline production with minimal energy and higher precision. Biochemical studies keep investigating D-forms, probing why some microbial systems build them in preference to L-forms, and how that quirk shapes evolutionary paths or drug design. Innovations in chromatography and mass spectrometry depend on stable, well-characterized reference materials—applications where D-(+)-Proline’s reliability makes lab work tick.
Lab rats and cell cultures rarely suffer pronounced effects from D-(+)-Proline, at least in concentrations typical for experimental work. Standard toxicological screening pegs it as minimally hazardous to humans and the environment unless exposures surge far beyond normal lab use. Key studies show D-proline does not fit into proteins of higher animals easily, so it doesn’t accumulate in major pathways. Its metabolic fate veers from the L-form; mammalian enzymes recognize D-amino acids poorly, which can offer resistance to peptidase digestion—a bump for pharmacokinetics in some drug designs. Even so, any novel derivative or high-dosage regime needs careful animal studies to rule out chronic impacts, as off-pathway metabolites could build up and stress liver or kidney function. Occupational safety sheets recommend keeping airborne particulates low and washing skin on contact. Persistent monitoring and evidence-based updates on safety guidelines remain essential, particularly once new proline analogs or larger scale uses roll out.
D-(+)-Proline stands at the intersection of synthetic chemistry’s shift toward greener, stereoselective methods. Demand for metal-free, high-precision catalysts grows as industries tackle cost, waste, and sustainability. Academic projects keep mining D-(+)-Proline’s scaffold for new organocatalysts with broader substrate scope and better performance under factory-floor conditions. Pharmaceutical development, looking to make metabolites tougher and drugs last longer, sees promise in D-amino acid residues—applications that may hinge on price drops and easier access to high-purity D-(+)-Proline. Fermentation-based production and enzyme engineering could close the loop, offering lower environmental impact and cost at scale. As regulatory pressures tighten, especially in Europe and North America, producers need tighter quality control and documentation to meet pharma and food standards. Whether in the next pharmaceutical breakthrough, a new material, or a more sustainable chemical process, D-(+)-Proline’s versatility and reliability keep it in the spotlight, driving both basic research and real-world innovations.
Walking through a chemistry lab, it doesn’t take long to spot proline somewhere on the shelves. D-(+)-Proline steps out from the crowd of amino acids, mostly because it shows up as one of those rare building blocks that curl the alphabet of biology in a different direction—it's the D-form, not the standard L-form used in human proteins. Most people don’t give much thought to the differences, but that tiny molecular twist changes everything for researchers, chemists, and even drug manufacturers.
D-(+)-Proline pops up in some unlikely places. Organic synthesis has claimed it for years as a trusted companion in making chiral molecules. "Chirality" might sound complicated, but in drug development, it makes or breaks whether a drug works in the body. D-(+)-Proline lends its structure to help chemists steer reactions down the right pathway, giving them products in high purity with the needed orientation. Chemists often talk about organocatalysis—using small organic molecules to speed up reactions—instead of relying on heavy metals. Here, D-(+)-Proline holds its ground, offering an affordable, non-toxic way to mimic enzymes in a flask that nobody needs to scrub metal out of later.
My time spent in the lab showed me firsthand how valuable this compound turns out to be, especially when making complex natural products. Fungi and bacteria produce both D and L forms of amino acids, but nature assigns each for a reason. Peptide drugs often need D-amino acids like D-(+)-Proline to dodge enzymes that chew up the L form too quickly. As a result, drugs containing D-(+)-Proline last longer and work better in the body. Some scientists even design new antibiotics with D-proline to outsmart bacterial resistance, while others use it in research to probe how cells tell left from right at the molecular scale.
On the industrial side, D-(+)-Proline finds its way into the manufacturing of specialty chemicals, pharmaceuticals, and food additives. Drug companies rely on it for making newer-generation antivirals and cancer treatments, especially where they look for stability and selective action. It helps shape the backbone of peptide drugs, changing how they interact in living systems. Companies exploring sustainable practices lean on D-(+)-Proline as a greener catalyst choice—no toxic metals, less waste, and easier product purification mean cleaner processes and better compliance with modern regulations.
Getting pure D-(+)-Proline costs more than scooping out the common L version. Most processes producing amino acids favor L-forms, making the D side struggle with price and supply. Scaling up without harming the environment or pushing up costs remains one hurdle. Biotechnology holds promise, offering fermentation with engineered microbes that churn out the D form at higher yields, cutting chemical by-products and slashing carbon footprints. As research funding catches up and demand rises, more companies adopt cleaner, bio-based production methods. Building strong partnerships between industry, academia, and regulatory agencies ensures that advances don’t stay stuck inside scientific journals but reach the hands that need them—researchers, medical professionals, and patients.
From reactions on the lab bench to the hunt for new medicines, D-(+)-Proline proves itself more than just another bottle in the chemical storeroom. Its role in shaping reliable, effective, and sustainable solutions deserves recognition, not only from the scientific community but from anyone who benefits downstream.
D-(+)-Proline belongs to the group of amino acids, the essential building blocks that shape the body’s proteins. D-(+)-Proline does its job with subtlety—it looks similar to its twin, L-proline, but its mirror-image structure tells a different story inside biochemical reactions. The chemical formula for D-(+)-Proline reads as C5H9NO2. Each molecule features a five-membered pyrrolidine ring; that bit is what sets proline apart from many other amino acids. Its carboxylic acid and amine don’t sit on separate side chains—the ring brings them face-to-face. That twist keeps proline rigid and, in the hands of a protein builder, it helps twist collagen into tight, sturdy coils.
Molecular weight matters, especially in laboratories where precision rules every step. D-(+)-Proline’s molecular weight clocks in at 115.13 g/mol. In the world of chemistry, these numbers guide how researchers scale up reactions, set up protein sequencing, or mix it into pharmaceutical syntheses. Even a few tenths of a gram can throw experiments off track, leave drug yields in the ditch, or trip up metabolic engineering projects.
The difference between D-(+)-Proline and its mirror image, L-proline, goes much deeper than appearance. Biochemistry relies on fit. Most enzymes built by nature recognize only one form, and proline's D or L form can be the difference between a successful biological function or a stalled one. D-(+)-Proline won’t step into most human proteins, but bacteria and fungi use it, and that means understanding both forms helps researchers track metabolic shortcuts or find new ways to disrupt harmful cells.
I remember helping a friend in the food industry troubleshoot a weird spoilage issue in cured meats. Some bacteria use D-(+)-Proline in their own amino acid cocktails—changing flavor, smell, and even the safety of aging meat. Knowing exactly what version of proline turned up changed how the plant adjusted its curing techniques and handled contamination.
In the pharmaceutical world, D-(+)-Proline takes on a second life. Some biologically active molecules rely specifically on the D form, rather than the L. Drug builders use D-(+)-Proline as a chiral pool precursor—a foundation that makes it easier to build molecules that target right-handed enzymes, resist breakdown, or hit unique disease targets. For researchers facing antimicrobial resistance, this amino acid provides one more pathway to trigger new defense mechanisms.
The flavor and fragrance industry finds its own use for D-(+)-Proline. It’s not just about building proteins—it’s about designing molecules that mimic or tweak sensory signals. That unexpected side of chemistry reaches the dining room, the chemistry lab, and sometimes even the doctor’s office.
Quality and traceability count. Many suppliers offer D-(+)-Proline with Certificate of Analysis, confirming purity by running advanced techniques like HPLC and NMR. Cross-checking structure and weight protects both researcher and consumer. Reliance on certified, well-documented sources supports trust, safety, and the potential for regulatory approval—crucial for both startups and established brands confronting global audits or expanding into stricter markets.
Whether in a clinic, a workshop, or a research institute, the details of chemical structure and weight shape results. Understanding exactly what D-(+)-Proline brings to the table empowers everyone from chemists through to food technologists and pharmaceutical developers. This is science grounded in detail, experience, and solid data.
Working with specialty chemicals like D-(+)-Proline reminds me of a few close calls back in the lab. Tiny mistakes added up. A little humidity here, a few degrees too warm there. Before long, a material that should have lasted six months dropped off in purity in half that time. In research and manufacturing, such breakdowns creep up silently, only to surface in poor results and unexpected costs.
Most powders coat themselves with invisible films of moisture if left outside the right environment. D-(+)-Proline fits right into this pattern. Even if it doesn’t clump right away, small changes in water content push the compound closer to decomposition. Moisture in the air enters through poorly sealed containers or even the tiniest openings. I once measured a batch that had picked up over 1% extra water in just a week sitting on a benchtop. While that may sound small, purity levels dropped enough to affect a peptide synthesis experiment. The result: hours lost, reagents wasted, and a team left troubleshooting instead of moving forward.
Heat always finds weak points in chemical bonds. Sustained exposure to room temperature, especially in non-air-conditioned storage spaces, speeds up D-(+)-Proline’s degradation. Research shows that dry, cool, and stable temperatures—preferably below 25°C—matter more than most appreciate. If a chemical fridge or a low-humidity room stands available, choosing those for storage keeps both product quality and sanity intact. At one supplier’s warehouse, products stored in upper racks near steam pipes routinely tested out of spec several weeks before open-date recommendations. The message landed: temperature consistency matters more than initial high purity.
Leaving these factors unchecked almost always brings surprises. Light, even indirect sunlight, triggers slow but steady breakdown of sensitive compounds. Though D-(+)-Proline isn’t as photo-sensitive as some, storing it in amber bottles or opaque containers creates another useful barrier. Air exposure seems harmless day to day. Oxygen, though, sneaks in to participate in subtle oxidation reactions over months. To solve that, I recommend working with small aliquots and returning bulk containers to their safe zone fast. Replace air in the container’s headspace with inert gas like nitrogen for longer-term storage, especially if the container gets opened frequently. Each exposure adds up.
Purchasing agents and lab techs deal with safety and shelf life every day. Skipping simple steps under the pressure of deadlines almost guarantees trouble down the line. Seal each container well and label opening dates. Rotate stock regularly so older supplies get used first. These habits save more money and frustration in one project cycle than most realize. If controls already in place seem overcautious, test a sample after three or six months and compare results—small changes in measured purity or appearance usually speak louder than emails or memos warning about shelf life.
Storing D-(+)-Proline dry and cool matters. Choose airtight containers, store away from light, and reseal quickly after use. Move open powders into a desiccator with a working drying agent inside. Short exposure to the workspace doesn’t spell disaster, but lazy habits build up losses over time. Respect for the details here comes from experience—each ruined batch, each delay in production, each unplanned replacement order. The safest storage isn’t just a formality—it’s the easiest way to keep the team focused on results instead of regrets.
Anyone who works in science or food tech stumbles across proline at some point. It’s a natural amino acid, found in collagen, and a basic building block for the human body. D-(+)-Proline is the mirror-image cousin of the form you get from steak or eggs. In nature, our bodies favor L-proline. Still, chem labs and industrial setups often look at D-(+)-Proline as a specialty ingredient.
D-(+)-Proline doesn’t show up on ingredient labels at grocery stores. The food industry only seriously considers amino acids that match our body’s own, and L-proline fits that bill. D-forms often carry risks—our biology hasn’t evolved to make much use of them, so the safety research just isn’t there. Players in food science stick to compounds with a clean bill of health and a long track record. For instance, some D-amino acids have cropped up in processed foods by accident, like in overcooked milk proteins, but regulators flag these as potential problems. With D-(+)-Proline, the food field finds little use. No direct benefit, and no health authority waving a green flag.
Pharma folks love building with amino acids. Drug design sometimes turns to D-amino acids to throw off enzymes and slow down breakdown in the body. But D-(+)-Proline comes with caution tape. There’s not much evidence pointing to unsafe outcomes at low doses, but absence of proof is no guarantee. Regulatory guidance—like from the FDA or EMA—leans toward decades of human evidence, and not much backs D-(+)-Proline for direct therapeutic use. In my own years reviewing supplements and therapies, the only proline listed on verified products stays firmly in the “L” camp. For D-(+)-Proline, pharma finds more action in test tubes than in pill bottles.
Step into a research lab, and the story changes. Chemical researchers use D-(+)-Proline to build chiral molecules—handed chemicals that fit together like left and right gloves. In the world of synthetic chemistry, creating the right chiral environment speeds up reactions and makes complex molecules possible. D-(+)-Proline acts as a catalyst or a building block for these specialized reactions. Think Nobel-winning chemistry, not grocery shelf products. Some labs also use it as a reference material to test for D-amino acid contamination in foods.
Lately, the conversation about unusual amino acids always circles back to safety, purpose, and proof. The food and pharma worlds both run on trust and regulation. Without clear evidence of benefit and a strong record on safety, D-(+)-Proline has no reason to show up in food products or drugs. In the research world, though, that same molecule unlocks doors in organic synthesis and analysis. It’s not about being good or bad, just about finding the right context. Scientists who know what they’re doing put D-(+)-Proline to work solving chemical puzzles, not building dinner or health products for the public.
If D-(+)-Proline ever lands a new role in wider industry, it will need public data, careful trials, and close looks from regulators. Today, most of its value lives inside research labs, far from the reach of everyday consumers. Anyone thinking about using this molecule outside controlled experiments has a long road of evidence to travel first.
D-(+)-Proline offers plenty of value in both research and industry circles, but few stop to think about what happens once the bottle comes off the shelf. It’s tempting to treat anything labeled as an amino acid with a casual attitude. After all, the word “amino” brings to mind vitamin bottles, not chemical hazards. But treating D-(+)-Proline with respect in the lab is just common sense. I’ve worked through enough chemical stains, coughing fits, and unexpected reactions to know that even mild irritants can sneak up on you.
For most tasks involving D-(+)-Proline, standard personal protection works best. Gloves—typically nitrile—help keep irritation at bay if the powder or any splashes get onto your skin. A well-fitted lab coat and safety goggles carry a lot of weight here too. The dust can find its way into your eyes if you pour or weigh it too quickly. Let’s be honest: plenty of us have shrugged off goggles in a rush, only to regret it seconds later.
A tidy workspace isn’t just about looking professional. Spilled crystals on a benchtop can haunt you days later if you’re working on unrelated projects. Keeping containers closed, labeling everything, and working in a vented area like a fume hood helps cut down accidental inhalation. It’s not about perfection—it’s about avoiding that “I can taste it in the air” moment. If you see dust floating while weighing out D-(+)-Proline, slow down, use a scoop, and seal things up promptly.
Proline holds up well in air, but humidity loves to clump fine powders. Stash it in a cool, dry cabinet away from heat sources—think away from sunlit windows and radiators. Moisture control packets make a difference, especially in older buildings where the weather creeps in. Proper labeling, including the hazard warnings, matters—especially if you work on a crowded shelf or share space with others. The one time I left a jar uncapped overnight, I found an unusable cake the next morning. Lesson learned: dry conditions win every time.
Handling a D-(+)-Proline spill rarely means panic, but you should sweep it up with gloves and minimize dust clouds. Dry towels or disposable cloths can help catch stray granules. Any significant contact on the skin? Wash off with soap and running water—nothing elaborate needed, just common care. For eye contact, rinse with water at an eyewash station for several minutes. If someone breathes in the dust and starts coughing, fresh air helps, and in rare cases, a visit to campus health or occupational medicine brings peace of mind.
Training new team members or students with D-(+)-Proline starts with patience and repetition. Explaining why gloves protect you or why airflow matters takes a few minutes, but it can save hours of trouble later. Safety data sheets aren’t bedtime reading, but glancing over the main points makes a difference. No one expects a spill or splash, but habits formed early keep people safe in the long run.
Some takeaways about D-(+)-Proline safety stick with you beyond a single experiment. Respect for chemicals doesn’t always come from a worst-case scenario, but from countless uneventful days where procedures just work. It fits into a bigger picture: attention to detail, basic care, and treating every compound—no matter how harmless it seems—as deserving of full attention.
| Names | |
| Preferred IUPAC name | (pyrrolidine-2-carboxylic acid) |
| Other names |
H-Pro-OH L-Proline S-(+)-Proline 2-Pyrrolidinecarboxylic acid (S)-Proline |
| Pronunciation | /ˈdiː pləˈriːn/ |
| Identifiers | |
| CAS Number | 344-25-2 |
| Beilstein Reference | 99955 |
| ChEBI | CHEBI:13212 |
| ChEMBL | CHEMBL1166 |
| ChemSpider | 108379 |
| DrugBank | DB00172 |
| ECHA InfoCard | 100.043.800 |
| EC Number | 214-895-7 |
| Gmelin Reference | 54438 |
| KEGG | C01776 |
| MeSH | D-Proline |
| PubChem CID | 61460 |
| RTECS number | TC6825000 |
| UNII | 8DUH1N11BX |
| UN number | UN3335 |
| Properties | |
| Chemical formula | C5H9NO2 |
| Molar mass | 115.13 g/mol |
| Appearance | White to almost white crystalline powder |
| Odor | Faint odor |
| Density | 1.04 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.41 |
| Acidity (pKa) | 2.99 |
| Basicity (pKb) | 10.64 |
| Magnetic susceptibility (χ) | -9.05 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.536 |
| Viscosity | Viscous liquid |
| Dipole moment | 8.47 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 59.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -589.5 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1946 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | A16AA22 |
| Hazards | |
| Main hazards | Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | No Hazard Statements. |
| Precautionary statements | P261-P264-P270-P272-P280-P301+P312-P330-P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 120 °C |
| Autoignition temperature | 455°C |
| Lethal dose or concentration | LD50 oral rat 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): >5000 mg/kg (Oral, Rat) |
| NIOSH | NT8050000 |
| REL (Recommended) | 1 g/L |
| Related compounds | |
| Related compounds |
L-Proline Proline Hydroxyproline |