Morpholine derivatives first drew attention decades back, after chemists noticed their stable ring structures and versatility in synthetic chemistry. Early studies into the 1960s led to the isolation of various isomers, including 2,6-dimethylmorpholine. Distinction between cis- and trans- forms took sharper focus as purification equipment improved, helping scientists tease apart unique stereochemical properties. This ability to control synthesis paved the way for targeting cis-2,6-dimethylmorpholine in the lab. Early producers saw steady interest from the pharmaceutical sector, as the molecule’s cyclic ether ring fit nicely into drug-scaffold strategies for enhancing solubility and biological compatibility.
Cis-2,6-dimethylmorpholine shows up as a colorless to pale yellow liquid, manageable enough in a standard lab. Its compact heterocyclic ring, decorated with two methyl groups at the 2 and 6 positions, gives it a slight edge in both steric and electronic properties compared with unsubstituted morpholine. Synthetic chemists value it for its high purity levels, minimal byproducts under standard conditions, and broad compatibility with varied reaction media. Low volatility keeps losses down during scale-up, which always pays off when working with expensive or tricky intermediates.
On the bench, cis-2,6-dimethylmorpholine carries a moderate molecular weight just above 115 g/mol. It dissolves smoothly in solvents like ethanol, ether, and even water, a trait handy for formulation and reaction work. Boiling point lands just above 175°C, so most evaporative loss isn’t a concern unless heating aggressively. The methyl groups tighten the structure, creating slightly more steric hindrance than its parent, with the cis configuration lending a bit of rigidity. Standard handling reveals limited odor and negligible vapor pressure at room temperature. The presence of secondary amine and ether functions opens pathways for both nucleophilic and electrophilic interactions.
Supplier labels roll out with typical information: CAS number, structural formula, purity percentages, melting and boiling points, and batch codes for traceability. Documentation also spells out moisture content, which matters for critical syntheses using anhydrous requirements. Commercial lots typically guarantee >98% purity, checked by GC and NMR, to rule out unwanted stereoisomers or hydrolyzed side products. Gross labeling requirements, covering potential hazards and recommended PPE, align with the globally harmonized system (GHS). Accurate labeling matters in my experience—mistakes here ripple into confusion in both synthesis and compliance routines.
The established route to cis-2,6-dimethylmorpholine starts with 2,6-dimethyl-1,4-dihalobutane, which undergoes cyclization with an ammonia source under controlled temperatures and pressure. Skilled chemists work in water, alcohol, or hydrocarbon solvent, introducing a base to mop up acid side products. Careful temperature control solves selectivity issues between cis and trans isomers. Flash chromatography or fractional distillation weeds out impurities, though on an industrial scale, continuous reactors and more sophisticated separation techniques hold sway. Tuning the cyclization conditions matters most, as trace contaminants from raw materials tend to gum up the works.
Cis-2,6-dimethylmorpholine behaves much like its parent morpholine under classic lab conditions, with a few twists from its substituents. The ring nitrogen’s lone pair gives the compound a strong nucleophilic bent, eager for alkylation, acylation, or sulfonation. The ether oxygen makes the ring more polar, letting it act as a ligand in coordination chemistry. With the cis configuration, approach angles for reactants change enough to influence yields, especially in asymmetric syntheses. For catalysis, attaching groups at the methylated positions can tune both solubility and steric blocking, while hydrogenation or oxidative cleavage allow functionalization off the core ring. Chemists stretch its applications with customized substituent swaps, often to build up more elaborate molecular libraries.
In catalogs or technical bulletins, cis-2,6-dimethylmorpholine sometimes turns up under less formal titles: 2,6-dimethyl-cis-morpholine, cis-dimethylmorpholine, or simply “cis-2,6-DMM." International suppliers periodically attach proprietary trade designations, especially for high-purity or pre-formulated variants targeting pharmaceutical or specialty chemical buyers.
Making safe use of cis-2,6-dimethylmorpholine means knowing the risks before the bottle comes off the shelf. Protective gloves and splash goggles help prevent eye and skin contact, as the secondary amine, while not exceptional in toxicity, can irritate mucous membranes with prolonged exposure. Airborne concentrations rarely hit harmful levels under standard lab ventilation, but chemical hygiene rules always recommend work under a fume hood. Storage calls for tightly sealed containers, away from oxidizers or acids, as morpholine rings sometimes react exothermically with strong reagents. Companies holding ISO 9001 or similar certifications fold these rules into site-wide safety manuals, drawing on data pulled from regulatory filings and accident reports.
Out in the world, cis-2,6-dimethylmorpholine proves valuable in crafting pharmaceuticals, especially where nitrogen heterocycles grant metabolic stability and improved solubility. Medicinal chemists draw on it as a tuner for side chain flexibility, important for designing new antidepressants and antiviral agents. Beyond drug space, it slips into specialty polymers and surfactants, where methyl substituents guide water compatibility or surface tension. Battery researchers and electronics firms find value in morpholine derivatives as electrolyte ingredients and corrosion inhibitors. In my lab days, custom syntheses often used cis-2,6-dimethylmorpholine as both starting point and intermediate for high-value products where small tweaks in molecular geometry led directly to better process yields or new activity profiles.
Search the literature, and you’ll see steady work on new reactions to manipulate this molecule’s core. Surface modifications, spinoffs for better drug transport, and novel coordination complexes fill patent filings and research journals. The focus lands on green chemistry: reducing waste during cyclization, swapping hazardous solvents for aqueous media, or squeezing higher yields through tricks like microwave-assistance. Cross-disciplinary teams—from academic chemists to pharma process engineers—dig into reaction kinetics, structure-activity relationships, and suitability as a fragment in combinatorial libraries. Plenty of ongoing work looks at alternative synthetic routes, aiming for less hazardous byproducts and cheaper starting materials.
Animal and cell culture studies paint a reassuring picture at typical lab concentrations, as cis-2,6-dimethylmorpholine rarely triggers acute toxicity in standard models. Still, no responsible safety assessment skips over chronic exposure. Some groups follow up on breakdown pathways, looking for metabolites after ingestion or environmental release. Existing toxicology data return low mutagenic and carcinogenic profiles, but regulators keep tabs on dermal and respiratory sensitivity—especially as industrial production ratchets up quantities. New safety data sheets reflect ongoing findings, updating exposure limits and personal protection factors. Recent years saw more effort in tracking environmental persistence, and scientists keep a cautious eye on long-term wastewater impacts.
Looking ahead, the chemistry of cis-2,6-dimethylmorpholine is set to move with technology in pharmaceuticals and materials science. Its adaptability means researchers can tailor modifications to meet rising demand for tailored drug scaffolds and specialty functional materials. As cleaner synthesis gains industry ground, attention shifts toward renewable feedstocks and solvent-efficient processes. Increased scrutiny on chemical safety, environmental impact, and occupational exposure will likely drive investment in upgraded assessment tools. Continued partnership between academic labs and industry can unlock new synthesis methods and unforeseen uses—sometimes the way forward depends on those experiments at the margins that try one new substituent, run one unexpected condition, and end up launching a whole new field of applications.
Chemicals like cis-2,6-dimethylmorpholine rarely make headlines, but they hold a steady presence in industries that rely on getting the little things right. At first glance, the name barely rings a bell for most people. In my own experience working alongside folks in chemical manufacturing, I've seen how certain compounds tend to become invisible even though they keep the wheels turning in a range of sectors. Understanding what cis-2,6-dimethylmorpholine brings to the table can point us toward smart, responsible use and open up conversations about safety, innovation, and health.
Cis-2,6-dimethylmorpholine steps into the spotlight in the coating industry, especially where paints and specialty coatings demand stability and durability. Paint formulators lean on it as a catalyst that helps speed up certain reactions, particularly when working with polyurethane and epoxy systems. It boosts reaction efficiency, so paint can harden and protect surfaces faster. Besides time savings, that means manufacturers can lower production costs without cutting quality, something I know businesses pay close attention to when margins get tight.
Rubber and plastic goods also owe a lot to this chemical’s performance as a catalyst. Think of things like insulation foams, molded parts, or shoe soles—industries depend on reliable curing and flexibility. Without proper catalysts, finished products crack sooner or fail to take shape correctly. Workers on factory floors often mention how small tweaks in catalyst choice can mean fewer production errors. With regulations keeping a close eye on material safety, companies gravitate toward additives with solid track records like cis-2,6-dimethylmorpholine.
Chemical synthesis in medicine sits squarely on the shoulders of various intermediates, and cis-2,6-dimethylmorpholine earns its stripes in pharmaceutical labs. Here, it helps build molecules that show up in everything from common medications to specialty drugs. The value rises with its purity, since pharmaceutical products need tight tolerances to protect patient health. It’s a behind-the-scenes role, but a crucial one, especially given the focus on drug quality and regulatory scrutiny these days.
Broad adoption also means paying attention to health and environmental impacts. Based on what’s known, proper handling and storage of cis-2,6-dimethylmorpholine go a long way in minimizing risks. My time spent in health and safety trainings made it clear: chemicals with niche uses can fly under the radar, and it falls to managers on-site to set up procedures that protect workers. Regulatory bodies require clear labeling and documentation, and updated safety protocols reflect lessons learned from past incidents in the sector.
With tightening rules on workplace exposure and product content, producers look for ways to improve processes without giving up performance. Some labs experiment with green chemistry to replace certain additives, others step up recycling or better waste management. Throughout my career, the most forward-thinking companies encourage feedback from both engineers and machine operators to refine their approach.
Smart choices with cis-2,6-dimethylmorpholine carry weight far beyond the lab or plant. As industries aim for safer, greener products, understanding what’s inside—or what makes production tick—is more important than ever.In the world of organic chemistry, a mouthful like “Cis-2,6-Dimethylmorpholine” tends to spark curiosity. The name tells us a lot about the molecule even before peering at a structure. Morpholine means a six-membered ring—think of four carbons, one nitrogen, and one oxygen all holding hands in a circle. Stick a methyl group on the second and sixth carbon, and choose the “cis” direction, so those methyls both poke up on the same side of the ring.
Let’s break down the numbers. The parent morpholine molecule uses the formula C4H9NO. Adding two methyl groups (each is CH3), one to carbon 2 and one to carbon 6, brings two more carbons and six hydrogens. This moves us to C6H15NO. Double-checking the structure can ease any doubts, but the sum stands: six carbons, fifteen hydrogens, one nitrogen, and one oxygen. The “cis” part doesn’t change the formula, just the 3D shape.
Chemical formulas sketch out the backbone, but in everyday chemistry, the arrangement makes a difference. “Cis” draws both methyl groups to the same face of the ring, and that changes how the molecule sits in the real world. Small tweaks like this can turn a compound’s behavior on its head. In pharmaceuticals, a cis or trans twist can lead to new interactions, sometimes boosting therapeutic properties, sometimes introducing side effects. That’s why chemists lean on both formula and configuration when studying molecules—structure guides function.
It’s easy to gloss over the impact of a humble heterocycle, yet these molecules shape everything from industrial lubricants to antidepressants. Morpholine derivatives often slip into solvent roles, corrosion inhibitors, and drug design. The methyl groups shore up the ring’s toughness and change its solubility and reactivity. Imagine swapping a trans isomer for a cis: sometimes, that simple shift unclogs a blocked reaction or reduces unwanted byproducts. In the real world, the difference between “works” and “doesnt work” often comes down to subtle geometry.
Industry folks like concrete answers. Laboratory catalogs reflect this: chemical suppliers list C6H15NO for both cis and trans isomers, qualified by naming. Checking public databases, such as PubChem or ChemSpider, lines up the numbers with real-world verification. The N-methyl morpholine family gets used in drug creation, plastics, and construction—each application benefiting from that tailored trio of atoms. Accurate formulas go beyond trivia; regulatory paperwork, shipping manifest, and safety sheet all start at the core identity of a molecule.
Here’s the rub: chemists stand at a crossroads of theory and practicality. A solid grasp of both systematic names and raw formulas avoids mistakes, saves time, and protects workers in labs or on factory floors. I’ve seen project teams lose days on a mix-up between two similar-sounding compounds. When details like “cis-2,6-Dimethylmorpholine, C6H15NO” show up clear and correct, everyone from researchers to logistics managers breathes easier. The chemical formula is no idle footnote—it’s a lifeline for safe and efficient science.
Cis-2,6-Dimethylmorpholine turns up in plenty of industrial labs, especially where companies need chemicals for synthesis. Some workers might run into it while manufacturing coatings or specialty chemicals. Its structure, with two extra methyl groups on the morpholine ring, makes it more than a boring building block—the stuff can carry risk if not given enough respect. It’s not just about “using it as directed” or “following standard protocols.” Each encounter matters.
You don’t spot warnings on data sheets by accident. A good look through reputable resources like the European Chemicals Agency or PubChem shows that Cis-2,6-Dimethylmorpholine acts as an irritant if it touches skin or splashes in eyes. Even a small dose can lead to uncomfortable reddening, stinging, or long-term sensitization with repeated contact. I’ve seen new lab techs underestimate gloves or eye protection, only to regret it after an accidental splash or spill. Inhalation represents another risk; vapors or aerosols can irritate the respiratory system. Even if someone feels fine at first, repeated careless exposure rarely ends well.
No one likes chemical burns or chronic allergy symptoms. Prepping for work around this substance, folks should pay attention to eye washes, chemical fume hoods, fit-checked gloves, and long sleeves. Lab safety isn’t just red tape from insurers; after a few chaotic incidents, you learn to trust good habits over short-term convenience.
Cis-2,6-Dimethylmorpholine lights up in the presence of ignition sources. Its vapors can produce hazardous combustion products, like nitrogen oxides. Chemical storerooms without good ventilation or proper signage put workers and firefighting teams in the crosshairs. I’ve seen small lab fires turn into big insurance headaches after a someone skipped grounding containers or locked the wrong solvents together. When people cut corners, the surrounding community ends up paying, not just the company involved.
Environmental consequences count too. Spills threaten waterways, as morpholine derivatives resist quick biodegradation. The effects can trickle into aquatic habitats—fish, microorganisms, or even local water sources might wind up in the firing line. Responsible disposal and spill kits ready to go make more sense than after-the-fact apologies.
Plenty of chemical incidents stem from folks who think, “It can’t happen to me.” Health agencies in the U.S. and Europe recommend annual safety refreshers focused not just on theory but on handling real spill drills, using proper PPE, and encouraging open reporting. Supervisors who cultivate a culture of open communication—where concerns can be aired without blame—see fewer small incidents spiraling out of control.
Some companies implement buddy systems for particularly tricky procedures, reducing both solo mistakes and that lurking urge to “get it done fast.” Proper storage—cool, well-ventilated, and clearly labeled—lowers the odds of cross-contamination or surprise vapor releases. Substitution with less hazardous alternatives stays on the table whenever chemistry allows it.
Cis-2,6-Dimethylmorpholine asks for vigilance. Regular, thoughtful handling and genuine commitment to safety signal respect for both colleagues and the larger environment. Cutting corners isn’t just risky for profits, it can carry serious health and legal consequences down the line. A culture fueled by training, responsible management, and honest communication shapes a safer place for everyone.
Every time I step into the chemical storage room, I remember that there’s no room for laziness or shortcuts. Cis-2,6-Dimethylmorpholine stands out as one of those compounds that asks for respect, not just caution. Over the years, I’ve seen labs where the biggest risks didn’t come from mysterious fumes or loud explosions, but from small bottles set on a wrong shelf, ignored too long under a hot vent, or capped just loosely enough to let trouble seep out.
Cis-2,6-Dimethylmorpholine isn’t a household name. Even seasoned researchers sometimes need to double-check its quirks before bringing it on site. Here’s the thing: this compound can react in ways that put both people and experiments at risk if it’s tossed onto a shelf without a plan. Vapors can irritate the lungs and eyes; skin exposure could trigger itching, redness, or worse with enough contact. The chemical may catch fire under certain conditions and its interactions with other substances can get unpredictable fast.
Air conditioning alone doesn’t cut it. Dedicated chemical refrigerators or temperature-controlled cabinets keep this compound in a sweet spot—cool, dry, and shielded from sunlight. Once, our department learned that lesson the hard way after a summer blackout: bottles that sat in a stuffy closet started leaking. Direct sunlight or steamy rooms turn minor risks into major headaches.
I always trust labels more than memory. Clear warning stickers, updated logs, and precise record-keeping help anyone working in the lab avoid guessing games. There’s no replacement for visual reminders on every bottle.
Cis-2,6-Dimethylmorpholine doesn’t play well with everything. I’ve watched enough new technicians learn that water, oxidizers, and acids nearby set the stage for disaster. Separate shelves, dividers, and even just reminders in storage SOPs stop mix-ups. Good storage turns into good lab habits, and nobody wants to explain why chemicals ended up mixed together on the news.
Some labs choose flame-proof cabinets, especially if bulk quantities come in. It doesn’t feel like extra work after the first few times—it’s like putting on a seatbelt, just part of the flow.
Spills can happen, no matter how careful everyone feels. Absorbent pads, gloves, splash goggles, and proper neutralizers take up little shelf space, but they let people act fast if bottles break or lids crack. I’ve learned it’s better to train every team member than to assume only supervisors will handle an emergency. A well-stocked spill kit by the storage area has saved a project more than once.
Routine checks catch leaks, broken seals, and outdated stock before they cause trouble. After every quarterly walk-through, more eyes catch what one person missed. Peer review doesn’t just work in publishing—it works in safety too.
Every new person on the team should walk through the storage setup before they ever touch Cis-2,6-Dimethylmorpholine. Shared checklists, safety data sheets on hand, and easy access to emergency numbers turn knowledge into action. Over time, I’ve seen trust and teamwork grow just by giving attention to such details.
Treating every bottle of Cis-2,6-Dimethylmorpholine with the gravity it deserves means fewer accidents, smoother research, and less worry all around. Safety isn’t an afterthought. It starts with good habits the moment that first shipment rolls in.
If you ever studied chemistry in school, you might remember the idea that a tiny shift in a molecule changes everything. The case with cis- and trans-2,6-dimethylmorpholine shows how real that lesson is. Both molecules look almost identical. Both spring from the same building blocks: a morpholine ring and two methyl groups glued at positions 2 and 6.
The real split comes from how those pieces twist in three-dimensional space. The “cis” structure puts both methyl groups on the same side of the ring, almost next to each other. The “trans” version sends them onto opposite sides, one sticking up and the other pointing down. This turns into two molecules with their own personalities and quirks, even though their written formulas match up exactly.
The reason this matters goes deeper than just shape. A chemist holding a beaker full of the cis isomer cannot just swap it for the trans version and expect everything to turn out the same. Even the scent, the melting point, and how easily the molecule dissolves in water may shift. The cis shape often crowds the ring, bumping the methyl groups together and putting stress on the structure. Trans, with its more balanced setup, tends to stretch out the ring more calmly. These shifts change how both isomers react with other molecules, or how resistant they are to breaking down in heat or under light.
This didn’t hit me until I worked on a project that meant coaxing a reaction along at low temperature. We banked on the reactivity of the trans isomer—it proved smoother to work with, less likely to clump up or misbehave as the temperature dropped. If we had tried the same with the cis form, side reactions often ate up our product, turning the day into a headache of troubleshooting. For anyone developing new medicines, these differences carry real weight: drug molecules often lock onto a target in the body with the same precision, and the wrong 3D arrangement will miss the mark entirely.
The difference between cis and trans isn’t just about lab life or obscure chemistry journals. Industries relying on particular reactions—factories making coatings or specialty polymers—sometimes chase one isomer for its predictable properties. Imagine investing in a process, lining up machines to churn out a certain product, only to discover you received a batch with the wrong isomer. Yields drop, purity slides, and the costs go up fast. Regulatory bodies such as the FDA pay close attention to stereoisomer content for these reasons, especially in pharmaceuticals, because a patient’s safety links directly to these minute details. The wrong isomer can be inactive—or even harmful.
Making sure to separate these isomers introduces extra steps and cost. We turn to methods like crystallization or selective catalysts to encourage the formation of one isomer over the other. Each of these methods depends on knowing exactly what shape your molecule takes and how it fits with catalysts or solvents. In my experience, ignoring these factors means wasted time and wasted materials. A sharper focus on separation and analysis methods—investing in reliable chromatography, refining synthesis protocols—can prevent a lot of headaches. Companies putting in the extra effort up front to get the right isomer see long-term stability and greater trust from their customers.
Paying close attention to the shape and arrangement of a molecule may feel old-fashioned in a world dominated by scale and speed, but it pays off in better science, cleaner results, and safer products. It’s easy to shrug off the need for this precision, especially when deadlines loom. Yet understanding the subtle differences between cis- and trans-2,6-dimethylmorpholine makes the difference between reliable outcomes and costly surprises. This lesson keeps showing up as the chemical industry keeps searching for smarter, safer, and more effective solutions.
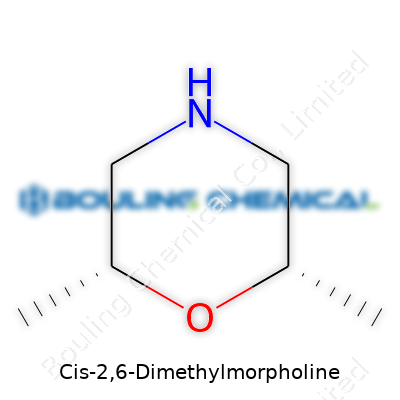
| Names | |
| Preferred IUPAC name | (2R,6R)-2,6-dimethylmorpholine |
| Other names |
2,6-Dimethyl-1,4-oxazinane Cis-2,6-dimethyl-1,4-oxazinane |
| Pronunciation | /ˈsɪs tuː sɪks daɪˈmɛθəl mɔːˈfəʊliːn/ |
| Identifiers | |
| CAS Number | 99199-60-7 |
| Beilstein Reference | 73458 |
| ChEBI | CHEBI:131848 |
| ChEMBL | CHEMBL2110648 |
| ChemSpider | 21559796 |
| DrugBank | DB04224 |
| ECHA InfoCard | ECHA InfoCard: 100.114.269 |
| EC Number | 219-136-8 |
| Gmelin Reference | 78750 |
| KEGG | C02104 |
| MeSH | D016693 |
| PubChem CID | 14327 |
| RTECS number | KL4025000 |
| UNII | 9W4H4T7D5F |
| UN number | 2735 |
| Properties | |
| Chemical formula | C6H13NO |
| Molar mass | 129.21 g/mol |
| Appearance | Colorless liquid |
| Odor | amine-like |
| Density | 0.927 g/mL at 25 °C(lit.) |
| Solubility in water | soluble |
| log P | 0.06 |
| Vapor pressure | 0.41 mmHg (25°C) |
| Acidity (pKa) | 9.85 |
| Basicity (pKb) | 4.51 |
| Magnetic susceptibility (χ) | -68.7·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.449 |
| Viscosity | 1.38 mPa·s (20 °C) |
| Dipole moment | 2.33 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 314.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -321.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4263.8 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H314 |
| Precautionary statements | Precautionary statements: P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 63.0 °C |
| Autoignition temperature | 370 °C |
| Explosive limits | Explosive limits: 1.3–9.8% |
| Lethal dose or concentration | Lethal dose or concentration (LD50/LC50): "Oral, rat: LD50 = 940 mg/kg |
| LD50 (median dose) | LD50 (median dose): oral/rat 670 mg/kg |
| NIOSH | SKC18060 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 3 ppm |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Morpholine 2,6-Dimethylmorpholine Trans-2,6-Dimethylmorpholine 2-Methylmorpholine N-Methylmorpholine |