Calcium compounds have always played a large role in daily life. In medicine, agriculture, and food, calcium salts crop up across centuries of research. Discovering new forms like Calcium Bis(5-Oxo-L-Prolinate) follows a common theme: chemistry builds on old building blocks, one tweak at a time, to fill new niches. The backbone of this molecule, proline, caught scientists’ attention thanks to its role in protein structures. Adding a calcium ion sparked a new chapter. Researchers in labs across Europe and East Asia began isolating and characterizing the compound in the early 2000s, driven by a search for better and safer calcium supplements and specialty reagents. Scientific journals such as the European Journal of Medicinal Chemistry documented its synthesis and unique properties, which set it apart from more generic calcium salts.
Most calcium supplements use familiar forms such as carbonate, citrate, or gluconate. Calcium Bis(5-Oxo-L-Prolinate) slides into a smaller, but significant market. Its use in the health supplement field remains growing, with manufacturers paying attention to its mix of bioavailability and compatibility with active ingredients. Some labs in pharmaceutical research test it for controlled-release formulas or as a carrier for specific drugs. Companies producing specialty chemicals take a close look at calcium amino acid chelates for everything from enhancing nutrition to improving stability in formulations. More than just another salt, it represents niche innovation, shaped as much by modern needs as by intermediates available from natural sources.
Anyone who’s handled calcium salts in a lab can tell the difference between a powdery carbonate and a fine, somewhat sticky amino acid chelate. Calcium Bis(5-Oxo-L-Prolinate) forms a fine, white to off-white crystalline powder. It dissolves readily in water, leaving little undissolved residue when prepared properly. The pH of a 1% aqueous solution tends toward neutrality, setting it apart from more acidic or alkaline choices. Molecular weight measures in the mid 300s. Its stability at ambient temperature suits both shelf storage and shipping, and moisture-absorbing tendencies mean it stores best in airtight containers, kept dry to avoid clumping or slow hydrolysis.
Chemical suppliers and food-grade manufacturers both rely on clear labeling and technical specs, which ensures safety in handling and lets buyers know exactly what they’re getting. Purity usually runs above 98%. Heavy metals, as always, remain restricted to parts-per-million or lower, and loss on drying tracks water content. There’s typically a COA—certificate of analysis—for every batch, spelling out trace impurities, particle size, and storage guidelines. Food applications draw close scrutiny, so labeling rules demand clarity: chemical name, batch number, expiration date, and handling instructions appear up front. Regulatory codes change from one country to the next. European REACH requirements or US FDA guidelines often mean extra paperwork, but the system is designed for clear product tracing and consumer trust.
It’s one thing to read a synthesis in a journal, another to reproduce it at scale. For Calcium Bis(5-Oxo-L-Prolinate), the most straightforward route uses L-proline, available in bulk, and reacts it with calcium salts under controlled pH, most often between 6.5 and 7.5 to encourage chelation without hydrolyzing either component. Careful titration of reactants, controlling reaction temperature (close to room temperature or under mild heating), and using pure water deliver a product that meets quality requirements. Filtration and drying steps ensure a clean, shelf-stable powder. Byproducts, usually minimal, get washed away with rinsing. Regular in-process testing by HPLC and other analytical methods keeps misbatches from reaching the finished product stage. Most production lines run in batches to keep contamination low and confidence high.
Chemistry in the lab rarely stands still. Calcium Bis(5-Oxo-L-Prolinate) can serve as a precursor for other materials, or act as a reactant in more complex syntheses. The oxo group invites nucleophilic attack, so chemists have tweaked the structure to graft on additional functional groups. Under alkaline conditions, mild oxidation produces derivatives useful in coordination chemistry. Its role as a chiral ligand crops up in asymmetric synthesis, where certain pharmaceutical drugs demand carefully controlled stereochemistry. In some nutritional research, scientists use it to form mixed complexes with trace elements, such as magnesium or zinc, to compare bioavailability and stability.
Industry never sticks to just one name. That’s why walking into a chemical warehouse in Shenzhen or Rotterdam, you might hear staff ask about calcium prolinate, calcium 5-oxo-prolinate, or even L-proline calcium salt. Some catalogs call it by alternative designations like Calpro, Prolical, or more obscure trade names used in proprietary blends. Chemical Abstracts Service assigns a number, but on the ground, buyers trust whatever local regulatory naming conventions require. This can cause confusion, so experienced handlers double-check paperwork and molecular structures, not just labels, to avoid costly mix-ups during procurement.
Lab safety officers take each new compound seriously, and Calcium Bis(5-Oxo-L-Prolinate) proves no different. Dust masks and gloves form the standard attire, since even low-toxicity chemicals can irritate sensitive skin or lungs. Its profile doesn’t flag major acute hazards, but eye contact or inhalation can trigger mild irritation. Workers in production environments stick to well-ventilated spaces, and larger facilities install dust collection hoods. Material safety data sheets flag the risks and proper first aid measures. Waste management routines require collection and neutralization with common agents before disposal—no skipping that step, even for “benign” chemicals. As production scales up, risk assessments encourage regular updates and drills, since procedures always change alongside batch sizes and facility layouts.
Pharmaceutical and nutraceutical manufacturers lead the push for applications. Calcium forms the backbone of bone health supplements, and chelated versions such as this one look promising for better uptake in the body, compared to rocky, chalky mineral forms. Some companies add it to infant formula or medical nutrition drinks, banking on its high solubility. It finds small but meaningful uses in specialty chemical synthesis, where its chiral character can play a role in drug development, and in agricultural testing, where new micronutrient delivery systems explore crop yield improvement. Its taste profile stays on the mild side, so it slips into fortification programs better than sharper-tasting calcium salts. In my own experience sourcing raw materials for vitamin blends, supply chain consistency remains key—shifts in crystal size or purity can throw off a production line.
In the world of food science and drug discovery, R&D teams pay close attention to chelated minerals. Universities and corporate labs run bioavailability studies to compare how much calcium the body really absorbs from this salt, versus older options. Early results show promising absorption rates in simulated digestion models, but clinical studies in humans remain underway. I’ve seen small-scale trials gauge its ability to improve bone health markers, alongside monitoring for side effects. Chemical engineers tinker with scale-up processes, hunting for new catalysts or reactor designs that push yields higher and cut costs. Patent filings continue apace, as companies hope to carve out IP protection for methods that smooth production or open up new applications. Collaboration between food technologists, pharmacologists, and materials scientists drives most of the real progress.
Toxicologists treat new ingredients with a heavy dose of skepticism, and rightly so. Animal studies check acute and chronic effects at several doses, and most testing flags calcium amino acid chelates as low-toxicity, provided the dose stays within recommended levels. Some research in rats and mice explores whether high daily intake might affect kidney or liver function. Results look similar to traditional calcium sources—overshooting the mark brings risk of hypercalcemia, but the compound itself doesn’t appear to introduce unique dangers. Genotoxicity screens clear it for food and pharma use at intended levels, but researchers still keep close watch for any rare allergic reactions or interactions with other medications, especially in sensitive groups like children or the elderly.
Future demand for specialty minerals rises as consumers become more educated about bioavailability and product quality. Calcium Bis(5-Oxo-L-Prolinate) fits into an emerging category of precision nutrition, where companies and researchers target optimal absorption—not just hitting a number on a label. As plant-based diets spread, interest in easy-to-absorb calcium alternatives jumps. Drug makers chase compounds that boost solubility and compatibility with active ingredients. In field trials, some ag-tech startups experiment with new forms for micronutrient-enriched hydroponics or animal feed. Further down the line, I see a push toward greener production methods that minimize waste and energy use—an important factor as climate impact rules get tighter. Real innovation depends on bridging lab curiosity with field-tested benefits, and that’s where this calcium salt may find its most important future role.
Calcium Bis(5-Oxo-L-Prolinate) rarely appears in everyday conversations, but in certain industries, it gets a lot of attention. In simple terms, this compound blends calcium—a mineral with a central role in bone health—with 5-oxo-L-proline, which stems from an amino acid important for body processes. This combination means it brings something unique to the table, especially in nutrition, pharmaceuticals, and biochemistry.
Years ago, I worked with a sports dietitian, and we spent hours reviewing new supplement formulations. Most people recognize calcium for keeping bones and teeth sturdy. Adding 5-oxo-L-prolinate isn’t just a gimmick for labels. Some research links this form with more predictable calcium absorption. Not every calcium salt works the same in the body: certain types pass through without leaving much of an impact. In contrast, the form found in Calcium Bis(5-Oxo-L-Prolinate) seems to help the mineral actually get into the system, rather than get flushed away.
Why would athletes or anyone active care? When bodies are under physical stress, efficient nutrient delivery becomes essential. Calcium shortfalls open the door to muscle cramps and weaken recovery after tough workouts. Formulations with this compound address those gaps in a practical way.
Drug makers hunt for compounds that make minerals more useful, both in tablets and injectables. Some calcium salts don’t mix well or even react with other active ingredients, causing headaches for formulators. The pairing with 5-oxo-L-prolinate gives a gentler touch, so medications mix more easily. This combination avoids harsh side effects and reduces irritation in sensitive patients or those dealing with gut issues. Easy handling on the production floor translates into more reliable products on pharmacy shelves.
Biochemistry labs treat Calcium Bis(5-Oxo-L-Prolinate) as a research tool. Every once in a while, the right compound reveals a lot about how the body works. Scientists use it to study calcium's journey through cells and tissues, since the prolinate element can tweak absorption patterns in controlled ways. I remember reading about one research project on neurobiology where this compound helped track metabolism in real time—something hard to do with older calcium salts. Students and researchers need options like this to make progress, whether they're designing cell culture experiments or running trials on nutrient delivery.
Calcium Bis(5-Oxo-L-Prolinate) doesn’t get splashy media coverage, but its potential goes beyond what sits in the supplement aisle. Manufacturers must keep checking for clear, well-documented studies that confirm its safety and long-term benefits. New regulations could encourage companies to disclose more data on actual calcium absorption rates in humans. Independent labs—rather than firms with a product to sell—should be the ones sharing those numbers.
Consumers deserve transparency about the real-world value of specialty calcium compounds. People should ask their healthcare providers before starting anything new, especially those with kidney or metabolic concerns. Clear labeling, consistent research, and open dialogue between makers, doctors, and consumers lay the groundwork for progress in the field. The story of Calcium Bis(5-Oxo-L-Prolinate) shows how chemistry and nutrition connect, touching everyday health in ways most folks may not realize.
We're surrounded by unfamiliar ingredients in supplements and processed foods. Calcium bis(5-oxo-L-prolinate) just sounds like another lab-born compound, but what does it offer, and how safe is it for daily use? Whenever something new lands on the health market, most people want clarity before letting it near their kitchens or their kids' diets.
Regulators like the Food and Drug Administration (FDA) in the US usually keep a close eye on new additives. They require manufacturers to provide scientific data about safety, potential toxicity, and overall effects on human biology. If you're buying a supplement, it's easy to get lost in chemical names and forget to look for that regulatory approval. Trust comes with transparency, so it matters whether this compound has made it through proper checks. Up until June 2024, it hasn't held "generally recognized as safe" status in many countries, and not much is published in peer-reviewed research for long-term health impacts.
Calcium bis(5-oxo-L-prolinate) brings both calcium — a crucial mineral for bones, nerves, and muscle function — and 5-oxo-L-proline, which plays a part in metabolism. On paper, this sounds like a good combo. Still, delivering calcium through a novel salt doesn't always guarantee better absorption or fewer side effects. I’ve talked to parents and nutritionists about why they stick with well-known sources like calcium citrate or carbonate: trust in tradition and a long safety track record. And for anyone worried about kidney stones or digestive upsets, unexpected calcium forms spark more concern than comfort.
Every new supplement ingredient arrives with grand promises: better absorption, fewer side effects, or some unique edge. There’s marketing, then there’s solid evidence. Published studies on calcium bis(5-oxo-L-prolinate) remain rare. Existing data mostly come from animal trials or unpublished manufacturer reports, which don’t tell us much about allergy risk, drug interactions, or long-term effects.
Real safety worries include the possibility of extra compounds changing how our systems handle calcium. Over-consuming calcium can raise risks for vascular calcification and kidney stones, especially in older adults. People with kidney disorders should always check with a doctor before trying something unfamiliar. In the absence of long-term safety data, the safer bet often remains traditional sources unless you face a specific medical need or a trusted professional recommends a switch.
Before letting novel ingredients into foods and supplements, more research needs to take place publicly — not just in industry labs. Studies tracking human health outcomes, digestive tolerance, calcium absorption rates, and potential allergies offer everyone much more peace of mind. Companies should also label products clearly, so consumers know exactly what’s inside and where it’s sourced.
Doctors and nutritionists play catch-up when new compounds enter the market with limited data. They deserve clear, unbiased information to guide people toward safe choices rather than just trusting glossy brochures. Everyone stands to gain from slower rollouts and full transparency — especially anyone feeding kids, the elderly, or those with chronic conditions.
Anyone considering a supplement with calcium bis(5-oxo-L-prolinate) should ask for batch-level safety data, check for regulatory approval in their country, and consult a healthcare provider. Here’s a simple test: Would you give this ingredient to someone fragile, like a child or an elderly parent, if solid studies don’t exist? If the answer is no, it’s worth waiting for more definitive research and regulatory guidance before trading in what’s already known to be safe.
Calcium Bis(5-Oxo-L-Prolinate) sounds like something you’d only run into in advanced chemistry classes, but its formula actually tells a lot about how it works and where it fits into science and industry. The core structure combines calcium, an essential mineral, with two 5-oxo-L-prolinate ligands. The chemical formula comes out to Ca(C5H6NO3)2. Looking into what this represents goes beyond numbers and letters—this connects to daily life, health, and how certain compounds make a difference in biological and industrial applications.
Each 5-oxo-L-prolinate comes from L-proline, one of the amino acids you’ll find in collagen, connective tissues, and food supplements. In this compound, proline transforms by losing a hydrogen and gaining an extra oxygen, which turns it into a '5-oxo' version. Two of these ligands then latch onto a single calcium ion, making this a chelate. Chelates pull double duty: they stabilize metal ions and help shuttle valuable minerals like calcium into places they’re needed. The formula also instantly points out the direct interaction between calcium and organic ligands, which influences how easily the body or a system can absorb and use the mineral.
I keep coming back to calcium’s role in daily life—bones, nerve function, muscle contraction. All these processes depend on reliable delivery of calcium, especially in forms the body recognizes. Traditional calcium salts can be tough to digest or poorly absorbed. Calcium Bis(5-Oxo-L-Prolinate), with its organic 'handle' in the form of prolinate, promises higher bioavailability. That means more calcium actually gets into your bloodstream and where it belongs. People with deficiencies or athletes looking to optimize can benefit from this difference.
Looking at actual nutritional studies and research, calcium compounds with amino acid chelates outperform normal, inorganic counterparts. For example, calcium bound to amino acids slips past some tough gut barriers, so tablets and powders using calcium bis(5-oxo-L-prolinate) work faster and more thoroughly. The real formula—Ca(C5H6NO3)2—directly enables this advantage because its structure shields the calcium and lets it pass through cell membranes more efficiently. Studies report fewer side effects like stomach discomfort or constipation, a common complaint with old-fashioned calcium carbonate supplements.
The same features that help in nutrition also attract attention in agriculture and pharmaceutical manufacturing. Chelated calcium feeds plants more efficiently by resisting precipitation in irrigation water, so farmers waste less and get better yields. In drug development, these ligands open doors to making calcium-based medications that behave predictably and safely in the body. The precise chemical formula helps chemists keep batch consistency, track purity, and troubleshoot production steps.
To push the promise of compounds like Calcium Bis(5-Oxo-L-Prolinate) forward, more transparency from manufacturers matters. Knowing the specific chemical formula means researchers and doctors can evaluate quality, trace sources, and compare effectiveness between products. Food supplement labels often hide behind generic names; clear chemical details let consumers and practitioners make informed choices. Public health relies not just on what’s in a bottle, but on whether it truly delivers benefits. Knowing your chemistry, especially in supplements, creates power for everyone who deals with health or science.
Sometimes, the basics get lost when folks handle specialty chemicals. On paper, Calcium Bis(5-Oxo-L-Prolinate) might sound like something you only worry about in a research lab, but the rules for keeping it stable are the kind anyone in the field has to respect. Mishandling storage pushes up the risk of degradation, brings safety questions, and makes valid results harder to guarantee. From past experience, the real edge comes from getting those fundamentals right, right from the start.
Calcium Bis(5-Oxo-L-Prolinate), like a lot of similar compounds, reacts badly to water. Exposing the material to humid air leads to clumping, breakdown, or, in the worst case, unsafe byproducts. Dry storage is step one—no shortcuts. Using a sealed container, ideally with a desiccant packet thrown in, keeps out ambient moisture. In one lab I worked with, a single night left open next to the sink ruined an entire batch: no one wants to repeat that lesson.
People sometimes stash chemicals out of sight and out of mind, but temperature swings and light exposure do quiet damage over time. Room temperature, away from direct sunlight, usually works best for Calcium Bis(5-Oxo-L-Prolinate). Keeping it inside a climate-controlled cabinet or a drawer makes a difference for longer-term shelf life. Even fluorescent lights have a way of kicking off slow degradation with the wrong kind of storage jar. Opaque or amber glass, used widely for more sensitive powders, earns its keep here too. If you want predictable results, keep exposure as low as possible—they found in several industry trials that light and warmth drop purity in months, not years.
Any seasoned chemist or technician could list half a dozen times cross-contamination made a mess of an experiment. Fragments of other substances can wreck both data and safety. Calcium Bis(5-Oxo-L-Prolinate) should get its own labeled container, never mixed with shared tools or left on communal shelves. Gloves and clean scoops—dedicated to the specific compound—keep things right. As simple as it sounds, this step keeps re-ordering costs way down and protects everyone in the workspace.
Even the best storage isn't worth much without good records. Labels should list the opening date, lot number, and the responsible user. More than once, I've seen teams track down a problem just by following the label trail. Rotation also matters—using up older stock before cracking open new shipments cuts down on accidental spoilage. Digital inventory systems might seem overwhelming, but once in place, they give peace of mind and speed up audits. This simple step means less guesswork and fewer mistakes for anyone reaching for the right jar at the right time.
Strong storage habits do more than meet compliance. They build a foundation for safe work, reliable results, and respect for everyone’s time and effort. Simple rules—dry, cool, dark, segregated, tracked—stop small oversights from becoming big problems. Years in labs taught me this: how you store the material decides what you can do with it tomorrow. So, keeping Calcium Bis(5-Oxo-L-Prolinate) under the right conditions isn't just protocol. It's doing the job right, every day.
Anyone handling chemicals in a lab, school, or factory knows the ritual: gloves, goggles, and a keen eye on the safety data sheet. Calcium Bis(5-Oxo-L-Prolinate) isn’t a household item. It calls for extra care. This material may come as a fine powder or granule. Small enough to go airborne, easy to miss. Inhaling dust or letting it touch bare skin creates avoidable problems—irritation, unexpected reactions, extra cleanup.
Basic personal protective equipment (PPE) keeps accidents rare. Well-fitted nitrile gloves and a lab coat protect skin. Dust masks or a well-rated respirator keep particles away from lungs. Eye shields guard against accidental splashes or flying dust. No shortcuts, no improvising with casual clothing.
It’s easy to underestimate the risks of substances with complex names and low toxicity. The danger with Calcium Bis(5-Oxo-L-Prolinate) lies in neglect, not explosive behavior. Many chemists lock up their acids and strong oxidizers while letting specialty salts sit in uncovered containers. Humidity, contamination, or simple forgetfulness can ruin a batch or lead to a surprise reaction.
Lab and storeroom good sense starts with solid labeling. Mark every bottle with the compound’s name, hazard details, and the date received. Keep materials in tightly sealed bottles, away from heat or moisture. Store separate from acids and strong oxidizers. If a spill happens, stop and clean it immediately with a damp (not soaking wet) disposable towel. No water flowing into drains. Small debris gets swept gently, double-bagged, and set aside for chemical waste.
What happens after use matters as much as safe handling in the lab. Pouring chemicals down the drain leads to long-term headaches for water treatment workers and the communities downstream. Even chemicals that seem low-risk can break down to produce unknown byproducts.
Most facilities collect unused Calcium Bis(5-Oxo-L-Prolinate) in clearly marked chemical waste buckets. Hazardous waste contractors handle these materials, often neutralizing or breaking them down before landfill or incineration. If the facility provides chemical waste pickups, share every detail—source, amount, storage method.
For people working alone or handling small amounts at home, there is no shortcut to proper disposal. Municipal hazardous waste events offer a trusted solution. Never dump chemicals on soil, storm drains, or in regular trash bins. Many cities encourage emailing or calling their waste disposal office to check on chemical drop-off events. Local laws differ, but nobody benefits from shortcuts.
Safe chemical handling proves reliability to coworkers, neighbors, and the environment. Public trust depends on open, honest documentation. Safety data sheets must stay accessible. Procedures for spills and accidents need to be posted on walls, within reach of anyone working nearby. New staff and students need real training, not just a binder full of theory.
The right approach comes from habit, not luck. In my experience, strong teams keep each other accountable, share safety tips, and shut down risky behavior before it costs anyone their health or credibility. Even with obscure chemicals, the basics always hold true: respect the risks, protect yourself, and think about where every gram will end up.
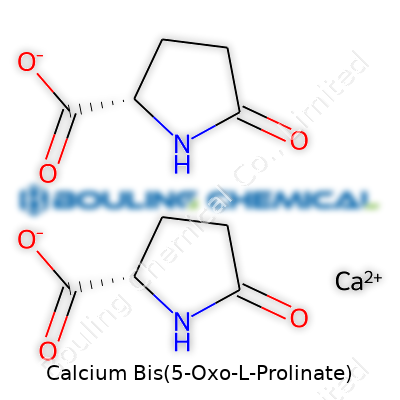
| Names | |
| Preferred IUPAC name | calcium bis[(2S)-5-oxopyrrolidine-2-carboxylate] |
| Other names |
Calcium bis(5-oxo-L-prolinate) Calcium pidolate Calcium pyroglutamate Pidolic acid calcium salt Calcium 5-oxo-L-prolinate Calcium 2-pyrrolidone-5-carboxylate |
| Pronunciation | /ˈkæl.si.əm bɪs(ˈfaɪv ˈɒk.səʊ ɛl prəʊˈlɪ.neɪt)/ |
| Identifiers | |
| CAS Number | 94338-40-2 |
| Beilstein Reference | 1758967 |
| ChEBI | CHEBI:85234 |
| ChEMBL | CHEMBL1233616 |
| ChemSpider | 23421870 |
| DrugBank | DB09294 |
| ECHA InfoCard | 03b0ef32-d6b7-43ed-94d8-fbc8b307590a |
| EC Number | 309-284-0 |
| Gmelin Reference | 871149 |
| KEGG | C18622 |
| MeSH | D-Calcium Bis(5-Oxo-L-Prolinate) |
| PubChem CID | 154109383 |
| RTECS number | NJ0843000 |
| UNII | 6D8H0K92FC |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID50866146 |
| Properties | |
| Chemical formula | C10H12CaN2O6 |
| Molar mass | 258.260 g/mol |
| Appearance | white to off-white powder |
| Odor | Odorless |
| Density | 1.5 g/cm3 |
| Solubility in water | Soluble in water |
| log P | -2.2 |
| Acidity (pKa) | 10.13 |
| Basicity (pKb) | 6.94 |
| Magnetic susceptibility (χ) | -61.5 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.570 |
| Dipole moment | 3.30 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 395.8 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | A12AA04 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | IF INHALED: Remove person to fresh air and keep comfortable for breathing. IF ON SKIN: Wash with plenty of water. IF IN EYES: Rinse cautiously with water for several minutes. If eye irritation persists: Get medical advice/attention. |
| LD50 (median dose) | LD50 (median dose) of Calcium Bis(5-Oxo-L-Prolinate) is **"5000 mg/kg (rat, oral)"**. |
| PEL (Permissible) | Not Established |
| REL (Recommended) | 0.01 mg/kg bw/day |
| Related compounds | |
| Related compounds |
Calcium gluconate Calcium lactate Magnesium bis(5-oxo-L-prolinate) Zinc bis(5-oxo-L-prolinate) Calcium ascorbate |