Chemistry has a habit of finding new tricks in old molecules, and borane morpholine is no exception. Back in the mid-20th century, scientists started looking for safer alternatives to plain borane. The pure stuff has a reputation for being both tricky and explosive. Chemists in the 1960s experimented by mixing borane with amines to get compounds that kept borane’s punch but dialed down its volatility. Morpholine stood out—not just because chemists had it lying around, but because it built a borane complex that worked better and traveled more safely. From early papers through to industrial scale-up, borane morpholine went from a risky lab curiosity to a commercial reagent. Along the way, technical hurdles turned into routine practices, letting researchers and industry handle it without so much white-knuckle chance.
Plenty of people have never heard of borane morpholine, but anyone who's had to reduce a carbonyl compound or tinker with a tricky organic synthesis probably knows its value. This reagent gets sold most often as a clear liquid or handled as a solution in tetrahydrofuran or toluene. Its shelf life depends a lot on how you store it—keep it away from air and water, and you can keep it stable for a good while. Chemical companies produce it for academic labs working on new pharmaceuticals, for companies building out their own synthetic pathways, or for those running up against old-fashioned reduction chemistry that needs a safer hand than sodium borohydride can offer.
At room temperature, borane morpholine holds up as a colorless to pale yellow liquid depending on how pure it is. Its molecular formula, C4H11BNO, means you’re looking at a relatively small molecule for something with big reactivity. Weight clocks in near 113 g/mol. The boiling point floats somewhere above 100°C, and borane morpholine barely mixes with water thanks to the nature of its bonds. It gives off a faint amine smell and can catch fire if you treat it carelessly. What sticks out is the B-H bond—it’s the ticket to the mild reducing properties that chemists like so much.
Buy a bottle of borane morpholine, and you’ll see a string of hazard labels. Flammable liquids warning, eye and skin irritant, and a Caution: moisture sensitive tag, always front and center. These warnings aren’t for show. The bottle will have the lot number, concentration (for solutions), expiration date, and storage recommendations, usually calling for a dry, cool spot under nitrogen or argon. Safety data sheets tell the rest of the story, including handling steps, emergency procedures, and disposal rules. That’s all before you open the cap and smell that subtle odor that tells you to double up on gloves and keep a fume hood nearby.
Large-scale synthesis means a tank of morpholine and a stream of diborane gas, all handled far away from open flames or even static sparks. The process usually involves cooling morpholine to slow everything down and control the exothermic reaction as borane slowly bubbles in. Industry methods improve yield by monitoring gas flow rates and temperature. In some setups, chemists use alternative boron sources or employ pressure reactors for better control. Lab-scale work gets away with simpler setups, but safety precautions never slip—diborane’s toxicity and flammability demand strict oversight from start to finish.
Borane morpholine finds a sweet spot in organic synthesis. It works reliably as a mild reducing agent—chemists reach for it to reduce carboxylic acids, esters, and amides down to alcohols without tearing apart sensitive groups elsewhere in the molecule. The morpholine doesn’t just act as a carrier; its electronic properties actually shape how the borane releases hydrogen for reductions. Other chemists have pushed the limits, modifying the morpholine or swapping it with different amines to adjust selectivity or reactivity. In cross-coupling reactions and asymmetric synthesis, some teams are digging into morpholine’s potential for producing chiral compounds. Nothing beats the satisfaction of finding a borane reagent that delivers reductions gently enough to leave the rest of a complex molecule intact.
Try ordering borane morpholine and you’ll see it under a handful of names. Some catalogs call it borane–morpholine complex or borane complex with 1-oxa-4-azacyclohexane. CAS number 3848-01-9 keeps confusion at bay. Vendor-specific labels sometimes shorten it to simply 'BH3-morpholine,' but it’s all the same stuff. Some companies focus on purity or packaging size, but no one mistakes it for anything else.
Working with borane morpholine means rolling up your sleeves and checking your routines. Lab coats, splash goggles, and nitrile gloves come standard. Fume hoods are a must since vapors can get out of hand without warning. No one wants open flames or static nearby; this is a flammable, moisture-intolerant liquid with a history of reacting violently if you don’t treat it right. Spill kits need to sit nearby, and disposal requires trained hands—waste gets neutralized before hitting ordinary streams. Repeated safety training keeps accidents low. A friend once told me about a graduate student who skipped protocol and nearly set off an alarm with a surprise reaction; after that, every lab meeting reviewed the same set of safety slides. So much of it comes down to paying attention and never rushing the prep.
Even outside pure chemistry labs, borane morpholine carves out space. Drug discovery leans on it in reducing steps during small-molecule synthesis. It enables routes unavailable by older reagents, especially where functional group compatibility counts. In material science, surface modifications benefit from mild reductions—no one wants surface chemistry to fail because a reagent worked too hard. Fine chemicals production and contract research organizations keep it in stock for these types of jobs, saving both time and yield loss. Some teams are even experimenting with using it in greener chemistry setups, trading its hazards for better control and less waste down the line.
Development moves fast in a field where minor tweaks can change an entire reaction’s path. Chemists have explored tuning the borane–morpholine ratio to nudge selectivity, and a few teams have looked at swapping out morpholine for related amines. Modern analytical tools like NMR and X-ray crystallography make it easier to see what’s changing on a molecular level, and some newer studies measure reaction rates in real time to find optimal setups. Machine learning even pops up occasionally, sifting through past experiments to steer future research towards higher yields and lower side-product formation. Patenting new routes often builds on tweaks to the borane morpholine process.
Borane morpholine isn’t as infamous as its parent diborane, but it isn’t harmless. Toxicity research shows it can irritate eyes, skin, and the respiratory system. Prolonged exposure has led to animal studies tracking organ effects, with results pointing to the need for careful industrial hygiene practices. Most of the focus falls on acute toxicity from mishandling or clean-up accidents. Chronic effects still get studied, especially as industry use grows. Teams producing borane morpholine watch air quality, require personal protective equipment, and monitor spills not just for immediate danger but also for left-behind contamination on benches or glassware.
Researchers always ask for more control, less waste, and safer supplies. Borane morpholine’s mild reduction ability puts it on lists for greener chemistry initiatives, where people want selective reactions with minimal by-products. Improvements in packaging and stabilization stand to make shipping and storage easier. There’s ongoing work looking for even lower toxicity alternatives, but borane morpholine holds on because of its predictable performance and ready availability. If new synthesis technologies keep shrinking environmental impact and boosting yield, future generations of this compound could show up in more processes, from drug design to complex material fabrication. The race to refine borane morpholine reminds me that chemistry never stands still—the same molecule can mean something entirely new once someone finds a fresh way to use it.
Walk into any research lab working with organic chemistry, and you’ll spot bottles marked with names like “borane morpholine.” These are not headline chemicals grabbing public attention, but for those who troubleshoot molecular puzzles, borane morpholine serves as a workhorse for selective reduction. Chemists like simple, reliable tools. Borane morpholine brings that to the table. In my experience, projects involving complex molecule synthesis quickly get tangled unless you have a way to trim down certain chemical groups at the right step.
In organic synthesis, reducing agents have personalities. Some bulldoze their way through every functional group, others work with a gentle touch. Borane morpholine falls into the latter camp. It handles reductions with a level head, making it valuable to chemists aiming to convert carboxylic acids to alcohols without mangling precious neighboring groups. Lithium aluminium hydride might overreact, sodium borohydride sometimes runs out of steam. Borane morpholine lands in the middle, ideal for situations that ask for care.
I remember a project where we tried building a new molecule for a drug synthesis route. Early rounds with sodium borohydride offered disappointing yields because the group we wanted to reduce just shrugged it off. Lining up a more aggressive reagent only risked destroying everything else. In this situation, borane morpholine provided a sweet spot. It dealt with the target group, skipped over sensitive parts of the molecule, and kept the process clean.
Applications go beyond graduate research projects. Pharmaceutical companies run large-scale reductions of functional groups, and they favor reagents that balance efficiency with selectivity. Borane morpholine helps transform carboxylic acids and esters into alcohols without sending neighboring groups into chaos. It comes in handy for fine chemicals, flavor synthesis, and when scaling up in industry.
The alternative, especially with older reduction agents, often means extra steps for protection and deprotection of groups that may otherwise get attacked. These extra steps waste time and money. Borane morpholine makes rooms for chemists to streamline, which tightens up timelines in industrial chemistry settings.
No chemical comes without risks. Borane morpholine deserves respect, not fear. It can give off toxic fumes, and improper storage sometimes leads to pressure issues. In the lab, we always cracked the fume hood open and avoided storing it with moisture. Good training and respect for the risks solve most of these problems.
Production also throws up concern about cost. A sharp spike in demand or interruptions in the supply chain can nudge prices upward. Labs and industry have learned to keep a close eye on sourcing and work out deals with suppliers to avoid sudden shortages at critical moments.
Borane morpholine won’t ever command front-page headlines. Yet, its steady use in research and industry reflects its value in the world of chemical synthesis. A reduction reaction made dependable can be the key to unlocking a breakthrough in drug development or a faster process for making specialty flavors and fragrances. That’s worth much more than quiet presence in a reagent bottle sitting on the shelf.
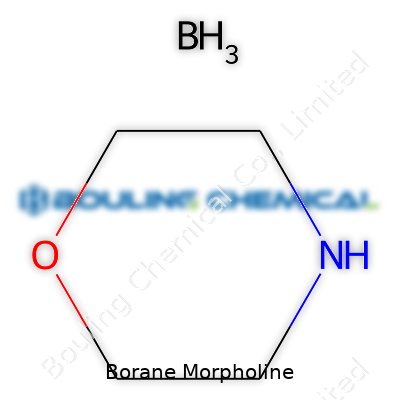
Borane Morpholine often enters the conversation in labs fixing up reductions or searching for milder reagents. Anyone dealing with organic synthesis—especially those untangling the time-consuming mess of reducing carboxylic acids—has likely brushed past it. The formula for this compound is C4H10BNO. Imagine morpholine, that classic six-membered ring with both nitrogen and oxygen sewn in, combined with a borane group. Put simply: morpholine (C4H9NO) meets borane (BH3), and they shake hands to make this concise, single complex.
I remember the first time I handled borane morpholine in a student lab. Bottles labeled “pyrophoric” always set me on edge, but borane morpholine, in its stable adduct form, took a lot of the fear out of reductions. Unlike borane alone, this stuff isn’t itching to burst into flames at the hint of air. That’s thanks to the morpholine, which locks onto borane like a seatbelt in a speeding car.
The structure looks simple but packs a lot of utility. Morpholine is a six-membered ring with nitrogen and oxygen opposite each other. In borane morpholine, borane snuggles right up to the nitrogen, forming a coordinate bond—nitrogen donates a lone pair, boron accepts. That lone pair-boron interaction gives the molecule its stability. Every chemist dealing with reactive boron knows how valuable that can be; borane by itself acts like a wild card, but morpholine tames it, letting people handle reductions more safely.
Chemically, this means the boron in borane morpholine carries three hydrogens, just like in borane gas (BH3). The nitrogen binds directly, and those hydrogens get put to work during reductions. I’ve seen it used to knock down carboxylic acids to alcohols and amides to amines—often with higher selectivity and fewer headaches than sodium borohydride or straight borane-THF. That matters a lot, especially during scale-up or in educational labs where safety sits above speed. Nothing ruins a morning faster than a runaway exothermic reduction, and borane morpholine makes that outcome less common.
Real-world labs—especially those working with sensitive, multi-step syntheses—need reagents that mix power with a softer touch. Borane morpholine fits this role by balancing reactivity with ease of handling. The structure means people can store and use it in less specialized glassware, without worrying the motion of air will set off problems. It’s also less likely to foam and misbehave, which I’ve found saves a lot of wasted time cleaning up glassware. Its shelf stability stretches budgets further, too.
Wider adoption gets slowed by cost; borane morpholine commands a higher price compared to other borane complexes. Some teams look for alternatives, or sometimes make compromises and stick with older, riskier reagents due to budget limits. There’s room for manufacturers to scale production and lower the price curve, opening access for more students and research teams not sitting on massive funds. Open-source protocols or shared synthetic routes for safe in-house preparation could also shift that balance.
Chemists can push for clearer safety data and better supplier transparency. Training sessions on handling reactive hydrides keep accidents rare. Government and industry grants aimed at making safer, more affordable reagents would open these doors even wider. Better collaboration between research institutes could spur novel morpholine-based borane derivatives, maybe even ones with specialty selectivities. Every time people use borane morpholine safely, that’s a signal that molecule-by-molecule, chemistry gets a lot easier—and a lot safer—for the next round of students and scientists.
Anyone who’s spent time in a chemistry lab knows there are substances you can almost ignore, and others that force you to sit up straight. Borane morpholine demands your attention. It’s a reducing agent that—if left open to the air, or even just sunlight—can break down or, worse, trigger real safety issues. No lab manager wants to fill out an accident report for something this avoidable. The truth: careless storage or sloppy handling puts both you and your work at risk.
Borane morpholine mixes flammability and reactivity in one package. Anyone who’s ever uncapped an old bottle will tell you—there’s nothing routine about it. I remember a time colleagues ignored peroxide-sensitive warnings. We spent half a day venting a hood and dealing with emergency protocols. This isn’t something you want to learn through experience. Its reaction with water can produce hydrogen gas—think about a spark in a lightly-ventilated prep room. That’s not a story you want to star in.
Forget tossing it on a shelf. Borane morpholine belongs in a stable, dry space, safe from temperature swings. Anyone serious about chemical handling keeps it out of sunlight and away from any moisture, using tightly sealed containers designed for harsh chemicals. Your everyday clear or thin-walled glass won’t cut it, and a plastic bottle sitting loose only invites trouble. In actual practice, a good desiccator or a sturdy metal can offers peace of mind.
Lab work always feels safe—until the day it doesn’t. Gloves, goggles, and a lab coat shouldn’t just be for photos in the safety training manual. Borane morpholine has a sharp, chemical smell that can hit hard; ventilation fans in fume hoods make a real difference. The best labs keep standard spill kits nearby, stocked with absorbent material and neutralizers. I’ve watched someone move too quickly, knock over a flask, and freeze in the moment. A prepared team, with training under their belt, gets through spills with less drama.
Some might shrug off disposal rules, thinking a diluted solution can just go down the drain. That’s wishful thinking. Local environmental regulators don’t take kindly to improper disposal of borane derivatives. The compounds can create flammable mixtures or pass harmful byproducts to water systems. Designate a compatible waste container, and hand it off to professionals—no exceptions.
Safe storage and careful handling aren’t just about following rules. They’re about protecting real people—yourself, your lab partners, even the cleaning staff who empty your bins. I’ve found that building checklists, scheduling regular reviews of storage areas, and running safety drills make everyone a little more comfortable and a lot safer. The real lesson: treat borane morpholine with respect, and you keep control of your workspace and your project.
Anyone who’s worked in a synthetic chemistry lab has probably heard stories about reagents that keep everyone on their toes. Borane morpholine is one of those. This compound helps chemists reduce a wide range of substances, making tough reactions smoother. Still, I remember my first time opening a bottle of borane morpholine—my mentor looked me dead in the eye and just said, “Gloves and goggles. Always.” She knew what she was talking about.
Borane morpholine isn’t out to help you; it’s ready to cause some trouble if you give it a chance. Its fumes aren’t friendly to lungs, and just a little spill on your skin can leave a mark you’ll remember. Here’s the kicker: in the presence of water, this compound reacts vigorously, releasing hydrogen gas. Once, a colleague got careless while transferring it and the solution bubbled over. We cleared the area pretty quick—nobody wants a flammable gas cloud hanging around.
Don’t take for granted that any splash will simply sting a little. We’ve seen technicians develop burns after brief contact. Breathing in even small amounts of vapor irritates not just the nose, but deep in the chest too. The “fishy” smell should serve as a warning sign—if you can smell it, you’re already getting exposed.
Never approach borane morpholine as if it’s no big deal. Every time someone let their guard down in my lab, it cost them. That’s why, for starters, a fume hood is the only reasonable place to use it. Even a small reaction can throw off vapors or foam up. Keep your gloves on at all times, and splash-proof goggles stay on your face, not your forehead.
Lab coats aren’t just for photographs, either. Those white cotton sleeves can save you a world of hurt if an accident happens. I’ve watched solvents eat through street clothes, but a lab coat at least gives you a few seconds to get cleaned off.
Water isn’t a friend here. Store borane morpholine in tightly sealed bottles, away from anything damp or humid. Clean up spills right away using proper absorbents—never water. I saw a rookie reach for a wet sponge, and our safety officer practically tackled him. No exaggeration, that sprint might have saved us a trip to the ER.
Keep the bottle away from acids and oxidizers. Hydrogen gas and a spark can add up fast to a nasty accident. Safe waste disposal matters, too: pour leftover material into designated containers, vented and kept cool until a professional picks it up.
Better training turned our team from nervous novices into a tight operation. Regular hazard talks, emergency drills, and giving everyone a voice kept us alert. New chemists paired up with experienced hands made mistakes rare.
It’s easy to forget how risky these reagents can be. Printed warnings fade into the background after a while, but sharing stories and seeing the scars up close kept us honest. Tough regulations aren’t there to make life harder for chemists—they’re written in scar tissue.
Borane morpholine isn’t a villain, just a tool with sharp teeth. With respect, practice, the right gear, and alert teammates, you get the job done and head home in one piece.
Work in an organic synthesis lab, even for a handful of projects, and you start to recognize reagents that make life easier. Borane Morpholine stands out among them. I remember reaching for it on the bench not because someone told me to, but because it helped me get through stubborn reductions that other reagents just complicated.
Borane Morpholine is probably best known for reducing carboxylic acids to alcohols. Chemists often bump into carboxylic acids that just don’t want to play nicely with milder agents. Sodium borohydride feels weak, and lithium aluminum hydride, while plenty strong, brings its own messes—pyrophoric hazards, tedious workups. Borane Morpholine steps in as a straightforward fix. It slices through the reduction cleanly without blowing up your project in a ball of drama.
I remember a project in grad school where we had to convert a benzoic acid derivative to a benzyl alcohol. LAH gave us low yields and endless headaches. Switched to Borane Morpholine after a professor’s tip. Overnight, our yields jumped and workup took half as much time. Its solubility in common organic solvents, and lower reactivity with water, means people spend less time worrying about reaction conditions.
Besides carboxylic acids, Borane Morpholine quietly scores in reducing amides to amines. Many amides thumb their nose at milder reagents, but Borane Morpholine keeps things tame enough to avoid harming nearby functional groups. In practice, this means making drugs or agricultural chemicals with sensitive molecules that wouldn’t survive harsher conditions.
It also handles esters efficiently, producing alcohols without a need for wild temperatures or extreme caution. This makes it a go-to tool in the semi-scale synthesis where cost and safety start to drive decisions. I’ve sat through group meetings where picking Borane Morpholine trimmed steps out of a route, saving both chemicals and wasted hours.
Borane complexes often intimidate people with their toxic gases or volatile nature. The morpholine version shapes up as a shelf-stable alternative. That shelf stability translates to fewer headaches in lab safety training and less stress for newcomers. Unlike the old BH3-THF solutions, Borane Morpholine won’t turn into a fire hazard if you forget about it for a week or two.
Scale-up chemists like it because the reagent handles concentration shifts and big glassware without treating every run like a risk. That dependability can change entire project timelines. I’ve seen colleagues able to skip safety redesigns on a pilot plant run because Borane Morpholine posed fewer hidden hazards.
Borane Morpholine isn’t perfect. It still carries some toxicity, and it doesn’t work for every functional group out there. People seek greener or less hazardous agents all the time. Catalytic hydrogenation or new enzyme-based methods promise cleaner syntheses. Yet for labs without access to fancy hydrogenation rigs or for those targeting molecules that tolerate little fuss, this reagent stays valuable.
Instead of swapping out every useful reagent at once, most labs focus on better ventilation, clear training, and alternatives for high-risk jobs. For now, Borane Morpholine remains a key tool, keeping reductions flowing when other options fall flat.
| Names | |
| Preferred IUPAC name | morpholinoborane |
| Other names |
Morpholine–borane complex Morpholine borane Borane morpholine complex |
| Pronunciation | /bɔːˈreɪn ˈmɔː.fəˌliːn/ |
| Identifiers | |
| CAS Number | 33343-35-0 |
| Beilstein Reference | 2738722 |
| ChEBI | CHEBI:39272 |
| ChEMBL | CHEMBL1302061 |
| ChemSpider | 71353 |
| DrugBank | DB11465 |
| ECHA InfoCard | 17b80b9f-b242-4373-8e58-083e4a3eb0de |
| EC Number | 217-118-8 |
| Gmelin Reference | 8772 |
| KEGG | C19184 |
| MeSH | D001919 |
| PubChem CID | 72813 |
| RTECS number | LQ8925000 |
| UNII | 4SGH6R711N |
| UN number | UN3461 |
| Properties | |
| Chemical formula | C4H12BNO |
| Molar mass | 113.98 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Ammoniacal |
| Density | 0.89 g/mL |
| Solubility in water | Reacts |
| log P | -0.47 |
| Vapor pressure | 0.3 mmHg (20 °C) |
| Acidity (pKa) | 8.80 |
| Basicity (pKb) | 8.8 |
| Magnetic susceptibility (χ) | -79.6 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.476 |
| Viscosity | 0.532 cP (20°C) |
| Dipole moment | 2.07 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 247.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -38.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4765 kJ mol−1 |
| Pharmacology | |
| ATC code | D08AX |
| Hazards | |
| GHS labelling | GHS02, GHS06, GHS08 |
| Pictograms | GHS02,GHS03,GHS05,GHS06 |
| Signal word | Danger |
| Precautionary statements | P210, P222, P231+P232, P261, P280, P304+P340, P305+P351+P338, P308+P313, P335+P334, P337+P313, P370+P378, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | Health: 3, Flammability: 4, Instability: 2, Special: - |
| Flash point | 36 °C (97 °F; 309 K) |
| Autoignition temperature | 210°C |
| Explosive limits | Explosive limits: 1.2–9.7% |
| Lethal dose or concentration | LD50 (oral, rat): 244 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Borane Morpholine: "131 mg/kg (oral, rat) |
| NIOSH | NA935 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Borane Morpholine: Not established |
| REL (Recommended) | TWA 0.5 ppm |
| IDLH (Immediate danger) | 100 ppm |
| Related compounds | |
| Related compounds |
Morpholine Borane-dimethyl sulfide complex Borane-tetrahydrofuran complex |