Benzyl Succinimido Carbonate didn’t land on laboratory shelves by accident. Years ago, researchers searched for better ways to introduce carbonate linkages into organic molecules. Back when pharmaceutical chemistry saw rapid growth in the early 20th century, chemists took inspiration from natural compounds that use carbamate groups for stability. The story of Benzyl Succinimido Carbonate follows the broader evolution of medicinal chemistry, where the push came from a need for more efficient, less toxic protecting groups for amines. I remember reading about early experiments where trial and error with different carbonate analogues led to improvements in yields and purity, opening doors for widespread adoption in both pharma and material science labs.
Benzyl Succinimido Carbonate is a crystalline compound built from a succinimide core, connected by a carbonate linkage to a benzyl group. Chemists value it for acting as a stable intermediate, allowing for selective transformations that would otherwise risk side reactions and degradation. In my experience, handling this molecule often feels safer than many related carbonates, thanks to its stability in storage and predictability in reactions. Large suppliers list it under different grades, sometimes meant for research, sometimes for industrial applications, underlining its versatility and demand.
In the lab, Benzyl Succinimido Carbonate appears as a white to off-white powder. It tends to be only slightly soluble in water, offering good solubility in common organic solvents like dichloromethane or acetone. Chemists recognize its relatively high melting point, often over 120°C, which makes transportation and storage less problematic for those without refrigerated storage facilities. Its carbonate functional group remains resilient to mild acidic or basic conditions, though strong alkali or acid usually triggers decomposition or transformation. Structurally, the compound's benzyl group helps with extraction steps and simplifies downstream processing.
Manufacturers commonly provide Benzyl Succinimido Carbonate with purity above 98%, backed by NMR and HPLC data. Labels usually carry risk and safety information under systems like GHS, noting the importance of avoiding inhalation or prolonged skin contact. Package sizes range from grams for specialty labs to kilograms for industrial buyers. Accurate batch records, production dates, and storage instructions come standard on each bottle, reflecting strict regulatory oversight in chemical distribution. Lab managers keep a close eye on lot numbers to ensure they track precisely which batches end up in which synthesis runs, protecting downstream data integrity.
To make Benzyl Succinimido Carbonate, synthetic chemists combine benzyl chloroformate with succinimide in the presence of a base. The base usually pulls off a proton from the nitrogen, making it reactive towards the carbonate. Early protocols called for careful addition of reactants and temperature control to prevent secondary products. Over the years, tweaks in stoichiometry, greener solvents, and better purification techniques improved safety and efficiency. Today’s processes often use less hazardous solvents and avoid excess reagents, cutting down on both cost and environmental footprint. Working in the lab, I found avoiding moisture essential, since any water can trigger side reactions and complicate the workup.
Benzyl Succinimido Carbonate acts as much more than just a stable intermediate. The carbonate group lends itself to nucleophilic substitution, such as when chemists want to attach the protective group to a sensitive amine. Deprotection can happen under mild hydrogenation conditions, removing the benzyl group and leaving the core carbonate in place. Structural modifications stem from altering either the aromatic ring or the succinimide backbone. In pharmaceutical development, slight tweaks in the molecule can tune how fast a drug releases its payload in the body or improve biological compatibility. A lot of trial runs in the lab revolve around balancing stability and reactivity for custom synthesis requests.
Trade catalogs and scientific papers don’t always use the same name for Benzyl Succinimido Carbonate. You’ll see listings under synonyms like N-Succinimidyl benzyl carbonate, Benzyl N-hydroxysuccinimidyl carbonate, and similar names depending on the registry or the tradition of the research group. Some suppliers assign unique catalog numbers and commercial brands, leading to confusion unless CAS number tracking is followed. Lab teams often keep a running list of synonyms to avoid order mistakes, since a single substitution could delay projects by weeks.
Working with Benzyl Succinimido Carbonate means following strict protocols. Safety data sheets stress the need for well-ventilated areas, lab coats, and protective eyewear. Accidental spills must be cleaned up quickly and disposed of in line with hazardous organic waste rules. As someone familiar with lab audits, I know inspectors always look for clear labeling, up-to-date documentation, and proper training around chemical handling. In large scale operations, engineers design systems to minimize airborne dust, since fine particulates can aggravate respiratory systems if inhaled over time.
The main action happens in pharmaceutical synthesis, where Benzyl Succinimido Carbonate helps chemists protect sensitive amino groups during multi-step reactions. Peptide synthesis teams reach for it to improve overall yields and cut down on purification headaches. Research on new polymers taps this compound for adding specific linkage points. Some diagnostic kits take advantage of its reactivity to label proteins or peptides with signaling molecules. On a practical level, Benzyl Succinimido Carbonate’s robust performance in high-throughput settings saves both time and money for contract research organizations under tight delivery schedules.
The last decade saw a burst of interest in optimizing carbonate reagents for green chemistry and drug development. Scientists keep exploring molecules like Benzyl Succinimido Carbonate to push bioconjugation techniques to new limits, whether for targeted drug delivery or vaccine development. Combining automation with new analytical tools made it possible to screen hundreds of reaction conditions at once, shortening the time from idea to functional compound. My interactions with people at research frontlines show continued tweaks in carbonate chemistry yield better selectivity and lower side-product rates, keeping labs competitive in a crowded market.
Toxicology studies show that Benzyl Succinimido Carbonate poses low acute toxicity at typical workplace exposure levels. Prolonged or repeated exposure, especially at high concentrations, triggers skin and eye irritation. Special concern arises for lab workers who may develop sensitization through unprotected handling. Environmental research traces the breakdown pathways in wastewater, noting that the compound degrades into benign end products after a few days. Still, chemical safety committees recommend paying close attention to both chronic exposure data and new findings on its breakdown products, since regulations adjust as evidence accumulates. From my discussions with occupational safety teams, regular retraining and clear signage build a safer workplace, since new hires and seasoned chemists both forget rules over time.
Looking ahead, Benzyl Succinimido Carbonate remains relevant as chemistry shifts toward more sustainable, less wasteful practices. Process engineers are chasing processes that cut solvent use or reclaim by-products more effectively. Specialty chemists are working on smarter derivatives, aiming to give even more precise control over reaction times and release profiles in drug delivery. As biopharmaceuticals gain ground, creative applications of carbonate chemistry could help bridge the gap between traditional small molecules and complex biologics. Industry and academia are investing in better predictive models for both safety and reactivity, giving development teams new ways to innovate while keeping risks in check. The big story comes from collaboration between sectors—where new ideas arise not just from technical need, but also from regulatory, environmental, and user demands on safer, faster chemistry.
Benzyl succinimido carbonate doesn’t turn up in daily conversation unless you’re working in a laboratory or reading the fine print on a pharmaceutical label. It’s a chemical compound with a big-sounding name, but its purpose is surprisingly practical. It’s all about making things work better, safer, or more efficiently, with most of its story rooted in the science of medications and advanced materials.
A lot of interest in benzyl succinimido carbonate comes from drug companies and research labs. This compound often shows up as a protecting group in the process of crafting new medications. Chemists use it to shield parts of a molecule they don’t want to react while they perform tricky chemical steps elsewhere. It helps keep other areas of those molecules stable and unaltered, so the final medicine does what it’s meant to do. When the time comes, the protecting group can get removed under carefully controlled conditions, so the drug’s structure lines up just right.
There are loads of examples showing the risks when you skip these careful steps. Impurities slip in. The drug doesn’t act consistently. Some medications fall short in stability tests, especially when exposed to heat, light, or oxygen. Benzyl succinimido carbonate helps prevent these problems by blocking pathways that might lead to unwanted by-products. Reliable medicines depend on this level of precision.
Benzyl succinimido carbonate doesn’t just stick to pharmaceuticals. It pops up in specialty chemicals and materials science as well. Manufacturers use the same trick—protecting sensitive parts of a molecule—to make complicated compounds without wrecking anything along the way. This reduces the number of reaction steps and cuts down on harsh or dangerous chemicals needed in manufacturing. That’s good for workers, and it also keeps chemical waste out of the environment, something that can’t be ignored today.
There’s another angle: producing safer ingredients for health care and consumer products. Chemists rely on benzyl succinimido carbonate to design new building blocks, especially when they can use it to make exact copies of molecules. Consistency means fewer surprises, less waste, and lower costs over time.
Good Manufacturing Practice (GMP) isn’t just a buzzword. Every step in drug production passes through strict controls to avoid cross-contamination or dangerous reactions. Benzyl succinimido carbonate adds an extra layer of security. Its role in reaction chains makes it easier to check quality by tracking changes at each stage. Regulators and safety experts like to see this level of accountability since it gives everyone confidence in a medicine’s safety record. People expect transparency, and tracking the journey of each ingredient helps deliver it.
As medical needs shift and chemists chase new types of molecules, the demand for precision tools like benzyl succinimido carbonate grows. Scientists continue developing safer reagents, greener methods, and new ways to reclaim or recycle by-products to answer tough questions about sustainability. Some companies push for alternatives with lower toxicity or greater biodegradability, hoping to make processes cleaner without losing performance. Partnerships between research labs, manufacturers, and environmental groups bring fresh ideas for safer, more effective ways to make the medicines and materials society counts on.
In my own experience, teams that pay close attention to every ingredient, not just the “main” ones, deliver products that work as promised. Customers notice the difference, especially those who depend on reliable results in critical situations. Benzyl succinimido carbonate might sound obscure, but the impact of careful chemistry reaches far into everyday life.
Benzyl Succinimido Carbonate isn’t a substance everyone talks about at the dinner table, but the chemistry behind it shapes how it gets used in the real world. To get to know this molecule, I look at its main parts. Start with the benzyl group. This is a string of carbon atoms capped with a six-membered ring, just like the backbone that gives many organic compounds their signatures. The benzyl unit gets attached through an ester linkage, which means there’s an oxygen bridge connecting it to the rest of the molecule.
Now, move to the succinimide. This part sets up a five-membered ring made of four carbon atoms and a nitrogen. Succinimide rings show up again and again in pharmaceuticals, often for good reason. Their stable structure lets them host other groups, like the carbonate in this case, without falling apart under mild conditions.
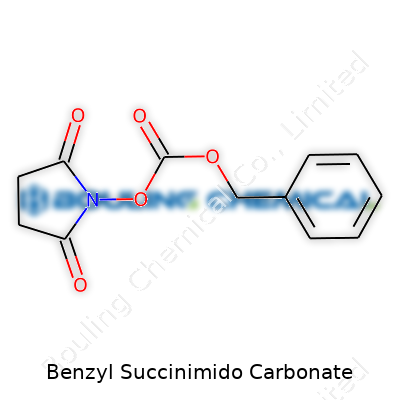
These pieces join up through a carbonate group, a linker known for its two oxygen atoms stitched to a central carbon. Chemists spot this carbonate by looking for its signature O–C(=O)–O arrangement. In benzyl succinimido carbonate, this group ties the benzyl and succinimido sections together. If you draw it out, you’ll see a benzyl oxygen pointing to a carbon, then two oxygens drawing the succinimido end into the mix. The sum of these features is: Benzyl-O–CO–N-succinimide.
The way the atoms lock together shapes not just the molecule’s “look,” but also how it acts. Carbonates break down under certain conditions, sometimes releasing the groups they link. That makes them useful for protecting sensitive drug components or giving off a molecule right where you want it — like a slow fuse on a delivery system. In pharmaceuticals and lab research, chemists commonly use benzyl carbonate and succinimido carbonate groups as protective masks to keep reactive parts under wraps until just the right moment.
Reading through published research, there’s a theme: detailed molecular design matters. For example, one paper in Journal of Medicinal Chemistry laid out how carbamate and carbonate linkers, like the one in benzyl succinimido carbonate, don’t just float around — they direct which bonds break during small molecule activation. That knowledge plays a big part in biotechnology and medical innovation, especially as labs create “prodrugs” that stay inactive until they drop their carbonate shield in the right place or at the right pH.
In my own chemistry work, I’ve found safety hinges on understanding these linkages. Carbonates can act as leaving groups, producing CO2 under the right triggers. That means handling, storage, and disposal need some attention. If care slips, breakdown products may show up in places you don’t expect. That’s where rigorous data, clear guidelines, and solid lab habits safeguard researchers and consumers alike.
Benzyl succinimido carbonate’s story highlights a broader lesson. Chemical structure isn’t just textbook knowledge — it’s the real reason compounds work as they do. When regulators or peer reviewers check new molecules, they want clear evidence of stability, breakdown pathways, and biological effects. Research journals and guidance from organizations like IUPAC ask for complete disclosure, with evidence based on reliable analysis. These efforts align with trusted science and transparent decision-making, giving medical teams, researchers, and the public the confidence to use innovative molecules safely.
Clear, accurate reporting on chemical structure supports safe synthesis and effective use. Labs that take the time to double-check their molecules, follow validated methods, and keep communication open between teams help make sure science serves real needs. That’s how new discoveries, including compounds like benzyl succinimido carbonate, can move from theory to trusted tools in medicine, research, and beyond.
Benzyl Succinimido Carbonate draws attention not from fame, but from its role in specialized chemistry labs and certain pharmaceutical projects. It looks like a pretty stable compound on paper, but make no mistake: ignoring storage guidance invites trouble. Mishandling leads to unpredictable breakdown and potentially dangerous reactions. I’ve seen hasty storage decisions cause headaches as bottles turn yellow and pressure builds up—no one wants to answer for that.
Room temperature in chemistry doesn’t always mean the same thing as at home. For stability, maintaining cool and dry conditions keeps Benzyl Succinimido Carbonate IN shape. From direct experience, ambient heat above 25°C pushes reactions along that nobody invited. Shelved close to a radiator or sunny window, the stuff loses its cool fast. Cold, consistent temperatures below 20°C—away from moisture—work in your favor.
Humidity is another enemy. One wrong move with a leaky stopper, and the contents start picking up water vapor, leading to hydrolysis. More than once, a neighbor’s shelf turned sticky because condensation got inside an unsealed bottle. Don’t stash it near sinks or dishwashers, even for a day. Desiccators lined with silica packs really prove their worth with these compounds.
Many compounds, including Benzyl Succinimido Carbonate, show their temper when left out in the open. If sunlight or harsh lab lights reach the bottle, photodegradation takes over. This process not only ruins the material, but also sets off a stench few forget. Opaque glass bottles help plenty—so does keeping them in cabinets with solid doors.
Air is another stressor. Leaving bottles open, even briefly during inventory, exposes the carbonate to oxygen and CO2. Screw caps—especially those with PTFE liners—protect way better than corks or simple snap-lids. Lock it up as you would with medicines you don’t want kids or pets to find.
Not all lab spaces fit every chemical. Smart storage means looking at compatibility lists and knowing which row won’t roast your carbonate. I worked with a technician who once stacked flammables with organic acids and regretted it after a night-time spill. Cabinets marked for “Reactive Organics” or basic “Cool Storage” offer the right isolation. Never mix the carbonate with oxidizers or strong acids, even temporarily.
Spill trays can catch leaks before they become nightmares. Whenever a bottle cracks or leaks, trays save cabinetry while containing the mess. In one incident, a tray meant the difference between a minor cleanup and a major hazmat call.
Durable, clear labeling helps keep everyone on the same page. Write the date opened, source, and hazard information right on the bottle. I’ve seen smart labs flutter for lack of decent labeling, then scramble to guess what’s inside and whether it’s still safe to use.
Regular inventory checks mean you spot trouble before it grows. Once a quarter, someone should inspect all bottles for signs of degradation, leaks, or crusty buildup. If you spot anything off, don’t risk it—dispose of the material following proper hazardous waste protocols.
Respect Benzyl Succinimido Carbonate and you’ll avoid unnecessary risks. Keep storage simple: cool, dry, sealed, and away from troublemakers. Use strong labels, keep up on inventory, and consult your chemical safety officer when unsure. Good storage habits don’t just check a box—they mean you come home safe after every shift.
Benzyl Succinimido Carbonate’s name feels like something straight out of a chemistry textbook, which always rings alarm bells for people who care about what goes into their products. Over the past few years, interest in so-called “hidden” chemicals has only picked up, especially in communities focused on wellness. Parents, workers, and consumers want fewer surprises in their food, cosmetics, and cleaning products. Folks always ask, “Could something like this sneak into my daily life and potentially affect health?”
Most people don’t have a flask of Benzyl Succinimido Carbonate sitting in their cupboards. If this compound shows up, it’s likely coming through indirect exposure—maybe as part of a specialized industrial process, a research lab, or rarely, as a component in complex manufacturing. Unlike everyday items like acetone or formaldehyde, it hasn’t made its way into household products where people could breathe it, touch it, or get it on their skin by accident. That’s a silver lining for anyone anxious about accidental exposure.
Looking through published toxicity studies and material safety data sheets reveals a gap. Most chemical databases keep quiet about Benzyl Succinimido Carbonate’s health impacts. For a trained eye, this usually means not enough independent study rather than guaranteed safety. Lack of data isn’t the same as safety assurance. Taking that into account, standard chemical risk management principles apply: use gloves and goggles handling any new compound, follow local safety rules, and don’t treat the unknown as harmless.
Personal experience working in labs has hammered home another key point: substances with a long and complicated name often deserve extra caution, even without a “toxic” label stamped across a data sheet. This wariness comes from years of seeing supposedly safe ingredients get reclassified after more rigorous tests. Health risks pop up when research moves past theory into real-world use. It’s surprising how quickly a material goes from “rare” to “everywhere” without consumers even knowing.
Absence of direct health warnings can’t erase all concerns. Scientists often look at compounds with similar structures to guess possible hazards. Benzyl-containing molecules sometimes irritate the skin or cause allergies. Succinimide derivatives have a mixed reputation, with some linked to nervous system effects and others labeled as benign. Until solid studies fill the gaps, it makes sense to lump Benzyl Succinimido Carbonate in the “handle with care” category.
People working with rare chemicals tend to rely on tried and true basics—proper labeling, clean workspaces, and easy access to material safety data sheets. Supervisors should encourage open questions about substances that look unfamiliar. For those outside lab settings, ask suppliers for ingredient lists and certificates if any exotic names appear in products. Consumers have more sway than ever, so pushing for transparency does help.
For companies considering new compounds, make health impact studies part of the launch process. That kind of diligence keeps workers safe and reassures everyone down the line. Government agencies and independent researchers should back deeper studies, making data available where regular people can find it—not locked behind expensive paywalls.
New chemical creations stretch the boundaries of what’s possible, but safety must keep pace with curiosity. Until scientists can say more, treat Benzyl Succinimido Carbonate with respect, just like you would any stranger you met in a new place.
Benzyl Succinimido Carbonate stands out in lab and industrial spaces where precision and creativity rule the day. This compound gets noticed by chemists who want to link molecules without stirring up trouble. Simple in structure, it gives researchers room for new reactions. Although the name feels scientific, its real value shows up in places where regular solutions fall short.
Drug research keeps pushing for smarter, more reliable shortcuts. Medicinal chemists face a big challenge: connect pieces together gently so delicate molecules stay whole. That’s where Benzyl Succinimido Carbonate enters the stage. Working at the bench, I’ve seen chemists pick this reagent for making specialized linkers. Instead of using harsh acids or tough conditions, the carbonate swaps out groups in a mild, predictable way. This helps when working with building blocks that get damaged under rough treatment.
Antibody-drug conjugates offer a clear example. These cancer drugs combine the sharp vision of an antibody with a toxic compound. The tricky part? Tether the two pieces without making a mess. Benzyl Succinimido Carbonate helps connect these parts. Its use helps ensure the drug reaches the target without falling apart. It also gives drug makers more control over how and when medicines break down in the body.
Innovation in materials science keeps picking up pace. People want new plastics, biodegradable polymers, and coatings that perform special tricks. Crosslinking reactions often sit at the start of these developments. The carbonate reacts cleanly with certain amines and alcohols, opening up new avenues to design smart polymers. Research teams can fine-tune their experiments because this chemical plays well with others and lets them swap out attachments predictably. In real lab work, this means fewer headaches and better results during scale-up.
Peptide chemistry comes with its own set of struggles. Making synthetic proteins or custom peptides sometimes feels like walking a tightrope—one misstep, and the chain falls apart. I’ve watched experienced chemists reach for Benzyl Succinimido Carbonate when they want to attach protective groups or add linkers without sacrificing yield. The reagent’s selectivity has helped bring down costs and raise the bar on purity. These gains matter whether a lab turns out research peptides or ingredients for clinical use.
Like many reagents, knowledge and care matter a lot. Working with Benzyl Succinimido Carbonate calls for personal protective equipment and good ventilation. Safety data sheets remind teams about risks to skin and eyes, as well as the importance of proper disposal. Companies following strict protocols lower the odds of exposure and keep chemists healthy. These habits echo the broader move toward greener labs and better stewardship.
Supply chains and environmental impact always deserve attention. Sourcing high-quality Benzyl Succinimido Carbonate supports safe and reliable research, but there is room for further improvement. Companies and research groups look for greener production routes and waste reduction. Greener chemistry isn’t just a trend; it is an answer to long-term challenges. Open conversations between manufacturers, researchers, and safety experts speed up positive change—shaping future labs for cleaner, safer work.
| Names | |
| Preferred IUPAC name | benzyl 1,3-dioxoisoindolin-2-yl carbonate |
| Other names |
Succinimidocarbonic acid benzyl ester Benzyl N-succinimidyl carbonate |
| Pronunciation | /ˈbɛn.zɪl sʌk.sɪˈnɪm.ɪ.doʊ ˈkɑːr.bə.neɪt/ |
| Identifiers | |
| CAS Number | 121980-60-5 |
| 3D model (JSmol) | `3D model (JSmol) string` for **Benzyl Succinimido Carbonate** (assuming the SMILES: `O=C(OCc1ccccc1)N2C(=O)CCC2=O`): ``` O=C(OCc1ccccc1)N2C(=O)CCC2=O ``` |
| Beilstein Reference | 3661394 |
| ChEBI | CHEBI:131556 |
| ChEMBL | CHEMBL297494 |
| ChemSpider | 25413715 |
| DrugBank | DB14675 |
| ECHA InfoCard | 03b20b27-1aad-425f-a252-fad01f9dcb1a |
| Gmelin Reference | 1260227 |
| KEGG | C18610 |
| MeSH | D003672 |
| PubChem CID | 154153895 |
| RTECS number | SY8575000 |
| UNII | 4580D3LC0G |
| UN number | UN3272 |
| CompTox Dashboard (EPA) | DTXSID50795456 |
| Properties | |
| Chemical formula | C12H11NO4 |
| Molar mass | 239.23 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.24 g/cm3 |
| Solubility in water | Insoluble |
| log P | 0.7 |
| Vapor pressure | Vapor pressure: 7.58E-08 mmHg at 25°C |
| Acidity (pKa) | 13.0 |
| Basicity (pKb) | 10.41 |
| Magnetic susceptibility (χ) | -62.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.572 |
| Dipole moment | 4.34 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 457.6 J/mol·K |
| Pharmacology | |
| ATC code | N07AB05 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | Precautionary statements: P261, P264, P271, P272, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P333+P313, P362+P364, P405, P501 |
| Flash point | > 140°C |
| LD50 (median dose) | LD50 (median dose): >2000 mg/kg (rat, oral) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.02 mg/L |
| IDLH (Immediate danger) | IDLH not listed |
| Related compounds | |
| Related compounds |
Benzyl chloroformate Succinimide Carbonyldiimidazole Benzyl alcohol N-hydroxysuccinimide |