Interest in 2-aminobenzothiazole goes back to a period when chemists drove themselves to push synthetic organic chemistry forward. Early academic labs in the late nineteenth century sought answers about aromatic heterocycles, looking for new medicines and dyes. Benzothiazole compounds took off after the 1900s, as sulfur and nitrogen in the same ring gave rise to a diverse set of reactions, leading to applications in both industry and research. Across the decades, 2-aminobenzothiazole attracted attention due to its handy reactivity and possibility for modification, each generation adding layers to the knowledge base.
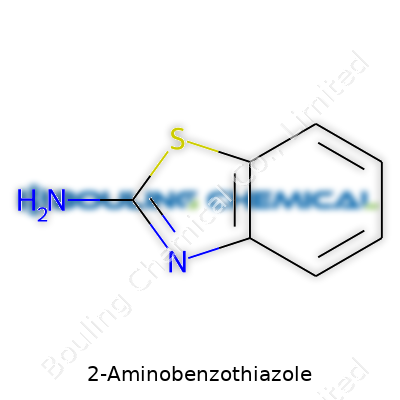
2-Aminobenzothiazole stands as an aromatic compound built from a benzothiazole backbone with an attached amino group at the second carbon. This orientation gives it a slightly electron-rich and reactive character, which underpins its utility in organic synthesis and pharmaceutical development. Chemical suppliers and research labs favor it for its versatility. As anyone who has spent time working at the bench knows, a bottle of 2-aminobenzothiazole opens up routes to more complex molecules, bioactive agents, and specialty chemicals.
Pure 2-aminobenzothiazole usually appears as a pale yellow to beige crystalline powder. It has a melting point hovering near 87 to 90 degrees Celsius, making it workable for most synthetic processes. Its molecular formula, C7H6N2S, and molar mass just under 150 g/mol mean it feels manageable for most laboratory and industrial uses. Solubility favors polar organic solvents, such as ethanol or dimethyl sulfoxide, while it resists dissolving in water at room temperature. A subtle sulfurous odor sometimes lingers in the air if the sample sits open for long. The amino group gives it nucleophilic characteristics, while the aromatic ring preserves stability during many transformations.
Anyone ordering or using 2-aminobenzothiazole expects clear labeling, listing CAS number 136-95-8, along with percent purity—often greater than 98%. Reputable suppliers specify batch number, lot analysis, storage recommendations, and supply Safety Data Sheets (SDS). In liquid chromatography, purity jumps into focus as a key requirement, with trace metal analysis important for pharmaceutical work. Labels include all essential hazard pictograms for workplace safety, and some packages show the preferred chemical structure for easy identification.
The main preparation pathway for 2-aminobenzothiazole draws from the cyclization of ortho-aminothiophenol with cyanogen bromide or similar agents under controlled temperature. Over the years, synthetic chemists have experimented with milder conditions, favoring liquid-phase reactions and greener solvents to avoid dangerous byproducts. In many labs, small-scale runs use polar aprotic solvents, while industry upgrades these to higher-yielding, less hazardous protocols. The emergence of microwave-assisted methods and flow chemistry systems shows that researchers keep looking for efficiency and reduced waste, ever alert for new catalysts and safer reagents.
2-Aminobenzothiazole excels as a building block. The amino group opens up coupling and substitution reactions, making it easy to introduce acyl, alkyl, or aryl groups. Electrophilic aromatic substitution happens on the benzene portion, often requiring careful control to avoid over-reaction. Diazotization and subsequent Sandmeyer reactions lead to halogenated derivatives, and sulfonation or nitration slots in broader substituents. Hydrogenation of the heterocycle reduces the core ring, offering approaches to saturated analogs. Over time, medicinal chemists have used this scaffold to build anticoagulants, antitumor compounds, and antimicrobial drugs. Polycondensation and fusion with other heterocycles spawn a wave of diverse structures in chemical literature.
Several alternate names circulate for 2-aminobenzothiazole, reflecting its multi-decade history and international handling. Examples include o-aminobenzothiazole, 2-benzothiazolamine, and Benzothiazol-2-ylamine. Catalogs and chemical databases link these synonyms, along with the standard IUPAC name, making literature searching and sourcing easier for chemists. Product labels also include structure diagrams, further reducing confusion for anyone who regularly works with multiple similar heterocycles.
Safety guidelines for 2-aminobenzothiazole call for standard personal protective gear: gloves, goggles, and lab coats. Inhalation and skin contact can cause irritation, and chronic exposure risks haven't been fully mapped out. Proper storage keeps the chemical dry and shielded from prolonged light, as moisture and heat may degrade quality. Fume hoods are standard during weighing and reaction setup because dust may cause sneezing or respiratory discomfort. Disposal through standard organic waste streams, using certified hazardous waste vendors, keeps regulatory boxes checked and coworker health protected. Training new staff on spill protocols and emergency eyewashes eliminates surprises.
Pharmaceutical research regularly turns to 2-aminobenzothiazole, thanks to its head start as a pharmacophore. Drug discovery pipelines explore it as a candidate in cancer therapy, antibacterial drugs, and as a core unit in enzyme inhibitors. Beyond medicine, agrochemical industries work it into fungicide and pesticide projects. Dye and pigment manufacturers rely on it for colorant production, especially where the aromatic thiazole ring offers vivid hues. Academic labs pick it for undergraduate teaching, demonstrating coupling reactions and N-heterocycle chemistry. Each field brings unique reactivity needs and leads to new byproducts, expanding the practical applications.
Current research into 2-aminobenzothiazole moves in several directions. Scientists search out derivatives that tackle drug resistance in microbes, driven by the urgency of health crises like MRSA. High-throughput screening tests hundreds of analogs for anti-inflammatory or anti-diabetic properties. Combinatorial chemistry benefits from its reactivity, supporting libraries of molecules for biological screening. Some projects in materials science look at how these compounds work in organic electronics or as components in advanced light-emitting devices. Grants and corporate partners invest where new applications promise both intellectual challenge and practical reward.
Toxicological studies show 2-aminobenzothiazole carries low to moderate acute toxicity in rodent models, but data remains incomplete on chronic effects or long-term environmental impact. Initial studies report liver and kidney effects at high doses, and some mutagenicity shows up under specific conditions. Researchers argue for full metabolic studies in humans, but regulatory agencies set guidelines on occupational exposure to keep risks minimal. Analytical chemists monitor workplace air content and wastewater outflow where production runs large. Environmental risk assessments continue, with available evidence pushing for careful use and improved handling standards.
The pathway ahead for 2-aminobenzothiazole looks busy. Medicinal chemists view it as a fruitful scaffold for next-generation antibiotics and anticancer drugs. As resistance continues rising, novel modifications of its core could offer new mechanisms of action. Green chemistry specialists build on recent gains, hoping to shrink hazardous waste in the manufacturing process. Analytical labs use the compound in sensor development owing to its electrochemical and photochemical behavior, tapping into the rise of portable diagnostics. The chemical’s rich reactivity profile promises more surprises as machine learning and automated synthesis open fresh possibilities in discovery chemistry.
The pharmacy shelf offers little hint about the chemistry powering common pills. Dig a bit, and 2-aminobenzothiazole starts showing up as a quiet workhorse. Medicinal chemists like it because the ring structure locks in stability, and the amine group opens doors to chemical tinkering. This combination lets researchers build more complex molecules, especially when fighting bacteria or cancer cells. Fluconazole, marketed for fungal infections, owes part of its backbone to this compound. The underlying story here is that the structure encourages strong interactions with certain enzymes—shutting down the bugs at the molecular level.
Farmers push against the relentless attack from fungi, bacteria, and weeds. Here again, 2-aminobenzothiazole shows up in the lab. Chemists design pesticides around this molecule to stop the pests while keeping the crops safe. The reason it crops up so often lies in its ability to disrupt vital biochemical processes in unwanted invaders, without leaving too many toxic traces behind. Today’s worries about resistance make this kind of targeted design even more valuable. Scientists keep tweaking the chemistry, hoping to stay a step ahead of pathogens that evolve quickly.
Anyone who’s noticed the long lifespan of car tires has seen chemistry in action. Vulcanization—the backbone of tough, lasting rubber—turned a corner with the discovery of accelerators based on benzothiazole structures. In formulas that cure rubber faster and make it much more durable, 2-aminobenzothiazole often serves as a starting material. It plays a role in setting up the conditions for sulfur and rubber to bond tightly. The improvements in wear, tear, and aging trace back in part to this synthesis work done decades ago. Factories favor these chemicals because they cut costs and improve safety in finished products.
Color matters in everything from clothing to printer ink. Certain hues only show up with help from molecules that absorb and reflect light in particular ways. Chemists add 2-aminobenzothiazole to dye recipes for its unique ability to tweak color properties. The structure interacts well with light, so the resulting dyes end up bright, long-lasting, and more resistant to fading. Textile industries find this valuable, especially where repeated washing is common. Since everyone notices worn-out colors fast, there’s always a push for pigments with a little more staying power, and this compound helps deliver that.
Labs testing for specific DNA or proteins often rely on molecular tags that glow under certain lights. Derivatives of 2-aminobenzothiazole pop up here too. Fluorescent probes built on this scaffold make it easier to spot disease markers. Researchers appreciate the clarity, speed, and precision that this chemistry can offer. Medical diagnostics benefit, and so do environmental monitoring programs that need to track minute traces of pollutants. The reason stems from how this molecule can join easily to other units while still holding onto its fluorescent charm. Every new discovery on this front opens up more accurate ways to see the invisible.
Seeing where 2-aminobenzothiazole fits in paints a picture of chemistry intersecting with daily needs. Practical benefits spill out, from healthier crops to better medicines and longer-lasting clothes. The pressure rests on researchers and regulatory bodies to watch for downsides like toxicity or environmental persistence, but experience keeps delivering better, safer modifications. So the next innovation in a medicine cabinet, a cornfield, or a laboratory may just owe something to this little aromatic ring, quietly moving things forward in the background.
Some chemicals grab attention for their role in medicine, manufacturing, and the scientific hunt for new materials. 2-Aminobenzothiazole stands as one of those boringly named compounds that quietly helps keep research labs ticking. The molecular formula, C7H6N2S, and the CAS number, 136-95-8, act like its digital fingerprint and home address in the chemical world. These numbers mean more than just bureaucracy. They keep orders consistent and lab shelves organized. Maybe that doesn’t sound thrilling, but a mix-up over numbers can cost a business a ton of money and put research at risk.
Mix-ups happen all the time in life: car keys, phone numbers, lunch in the fridge. In chemistry, a small mistake can mean a ruined experiment or mislabeled hazard. The CAS number works like a Social Security Number for chemicals. You can call someone by name, but if the name is not unique, things get confusing. 2-Aminobenzothiazole only means something if every order, shipment, or research paper points to the right substance, not just something that sounds close.
From my time digging through old lab reports, I've seen what happens when someone jots down a name without double-checking the CAS number. People have wasted days, even weeks, on tracking down the right material. Shipping the wrong stuff isn’t just annoying, it brings lab work to a crawl and sometimes throws safety out the window. Getting both the formula and the CAS right saves headaches and helps new discoveries stack up instead of stall out.
The compound itself pulls weight in lots of chemistry projects. It plays a big role in making dyes, pigments, and some medicines. As companies push to create better antibiotics and chase up sustainable materials, compounds like these act as springboards. A little bit of the right base molecule can open the door to long-lasting coatings, disease-fighting drugs, and plenty of everyday stuff that people don’t think about.
Any time research tries to develop something new with a thiazole ring, 2-Aminobenzothiazole gets mentioned in the patents and the formula sheets. Without a way to quickly and accurately signal exactly what’s being discussed, the whole process would bog down. Sloppy identification leads to wasted money and sometimes dangerous mix-ups, especially for companies trying to play by international safety rules.
A lot of errors in a lab don’t come from fancy machines or fickle chemistry—they come from people, paperwork, and labels. I’ve seen scientists spend more time arguing over which batch number matches which vial than actually doing science. For companies, the cost isn’t just time, but sometimes entire product lines. As labs shift toward automation and computer-tracked inventory, there’s less room for error—so sticking that CAS number on every sheet and ordering form becomes second nature.
Some firms have started using barcodes tied to CAS numbers, making tracking and inventory tougher to mess up. Others put up checklists and require sign-off for every new delivery. Each of these habits cuts down on the risk of swapping one compound for another. One simple digit in the wrong spot, and somebody might rerun months of work. The habit of double-checking numbers begins with the basics and proves itself every single day.
Getting better at tracking and identifying chemicals means more than shaving minutes off a checklist. It stands for safety, reliability, and keeping research honest. Instituting clear rules to always note CAS numbers alongside names builds muscle memory that shields against costly slip-ups. Crowded laboratories, supply chain hiccups, and the global hunt for new medicines all benefit from this level of discipline.
Anyone working around chemicals long enough picks up a few basic principles: keep things neat, don’t cut corners, and know what you’re dealing with. 2-Aminobenzothiazole doesn’t come with bells and whistles, but it demands respect, just like every chemical with a track record in pharmaceuticals and dyes.
Leaving 2-Aminobenzothiazole lying around without care can lead to headaches—literally and figuratively. The stuff tends to irritate skin, eyes, and lungs. Personally, I always picture a dusty jar tucked under a leaky shelf; it’s a disaster waiting to happen. The safest bet involves keeping it in a tightly sealed container, away from sunlight and moisture. Temperatures stay cool and stable in a dedicated chemical cabinet—think of it like coming home to a steady room, nobody wants their environment wild and unpredictable.
Stack storage containers on sturdy, labeled shelves. No one wants that unpleasant surprise of a cracked bottle from a careless bump. Glass works well, though plastic can do the trick as long as it doesn’t react with the substance. Check compatibility charts before tossing powders or solutions into any old jar—nothing shortens careers like surprise chemical reactions.
Familiar gloves and goggles don’t feel optional, even for seasoned hands. It’s tempting to skip extra steps for a quick job, but one splash or whiff changes minds fast. Nitrile gloves and eye protection keep things comfortable, even after hours on the bench. If the powder seems particularly floaty or fine, a dust mask helps cut down on accidental inhalation.
Work inside a fume hood whenever possible. I’ve watched colleagues shrug off small spills because they think it's “just a little powder.” A hood pulls irritating dusts away before anyone has to find out what a burning throat feels like. From personal experience, once you’ve cleared up a spill before lunchtime, it’s clear how a little extra care spares trouble later.
Benzothiazole-based compounds don’t belong in the trash or down the drain. Most labs keep a waste drum specifically for organic chemicals, and adding waste to the right container stops a lot of problems before they start. If a spill does happen, don’t try to sweep it dry—grab the right absorbent material and keep your gloves on. Sweep up the mess gently, then wash the area with just enough water and detergent. Forget the idea of “good enough”; loose powder and residue have no business sticking around.
Handwritten tags or fading stickers invite mix-ups. Everyone in the lab benefits from clear, permanent labeling. If there’s ever a transfer to a smaller bottle for weighing or transport, slap on a fresh label before setting the container down. The extra second spent now pays off big, especially when someone new moves through the workspace or cleans up at the end of a shift.
No matter how many protocols exist, they’re wasted if folks don’t follow them. Stories from coworkers prove it: someone skips orientation, someone else learns about benzothiazole by looking it up after feeling queasy. Regular training and reminders keep safety in focus, not just on paperwork but in everyday practice. If everyone chips in, the chemical stays just another tool, not a hazard waiting in the wings.
Working with chemicals like 2-Aminobenzothiazole brings an odd mix of familiarity and caution. I’ve pulled many jars from chemical stockrooms—labels faded, caps crusted. You forget, for a moment, how the smallest lapse matters. Still, any organic compound, especially something with a benzothiazole ring, grabs my attention. Maybe it’s the slightly fishy smell or the stubborn powder that escapes with a cough on the benchtop. You learn quickly—gloves are not optional, and open containers deserve respect.
I remember stories from old supervisors about eye splashes and ruined lunch breaks. 2-Aminobenzothiazole doesn’t light up a hazard chart the way concentrated acids do, but ignoring its risks makes no sense. Direct skin or eye exposure often leads to irritation. Prolonged contact can cause redness, itching, or a rash. Dust inhalation is not just a worry for people with asthma; even a robust set of lungs feels the effects after an accidental inhale. According to the European Chemical Agency, repeated exposure may trigger allergic reactions. There’s credible talk about liver and kidney trouble with chronic high-level exposure.
Some people have a habit of storing bottles anywhere with room. This can be a big mistake. 2-Aminobenzothiazole itself isn’t wildly flammable, but at elevated temperatures, it decomposes and releases toxic fumes—think sulfur oxides and nitrogen oxides. Anyone familiar with summer lab days knows how quickly a stuffy storeroom can escalate into an emergency if we’re careless. Tossing the residue down the drain might seem harmless, but nature doesn’t forgive shortcuts. This compound’s fate in rivers or soil isn’t well documented, so lab protocols treat it as a potential pollutant.
The simplest habits always pay off. Fresh gloves, splash-proof goggles, and working with powders under a fume hood—none of these steps are for show. They kept me—and everyone in earshot—safe after a spill. It’s never just about the one person handling the jar. Keep the powder out of open air. Wipe benches with damp rags to trap stray particles.
Proper storage matters. Keep 2-Aminobenzothiazole tightly sealed in a cool, dry cabinet. Mark the bottles boldly and never mix with oxidizers or strong acids. Clean up every crumb of residue and treat wipes or empty vials as hazardous; they go into proper chemical waste, never in everyday trash. According to OSHA, routine safety drills and chemical spill plans save both money and skin, so I would never skip refresher training. If something spills, call a supervisor—don’t play hero.
No one has a perfect record with laboratory safety. We mess up, and sometimes that’s the only way lessons stick. Emphasizing routine checks, proper gear, and real dialogue about what can go wrong makes a bigger difference than a stack of warning posters. 2-Aminobenzothiazole is just one example. The broader message applies to every chemical bottle on a shelf: if you treat safety as an afterthought, any compound can teach you a harsh lesson.
Anyone who’s handled chemicals knows purity isn’t just a technical term—it’s what separates a successful synthesis from a batch of unpredictable junk. 2-Aminobenzothiazole really puts this lesson into practice. There’s the typical lab grade, with purity above 98%, you see in research settings. That’s what I’ve used in grad school synthesizing new compounds. Even a percentage point drop introduces weird by-products that muddy your results.
Outside research, there’s production-grade material for broader industrial applications. These batches, though a little less pure, often do the trick for dye manufacturing or intermediate steps where pin-sharp accuracy isn’t critical. Skimping on purity in fine chemical work does nobody favors. But for larger-scale jobs, paying for the highest specification doesn’t always make sense.
There’s a reason vendors offer 2-Aminobenzothiazole in everything from small bottles to multi-kilo drums. In my experience, the fifty-gram bottle suits academic labs running a few reactions. Bulk options cater to companies churning out tons of product. Small quantities keep waste down and cut inventory headaches. Nobody wants to pay hazmat fees and store leftovers unless they absolutely must.
I remember one project where our department pooled resources just to split a small batch because we could barely get through tens of grams a semester. Across the hall, the industrial partners barely batted an eye at multi-kilo orders because cost per gram dropped. That’s why scientific supply houses get creative with packaging: tiny amber vials for shelf stability, larger HDPE jugs for users burning through their stocks.
Drums get strapped onto pallets for manufacturers, while research teams stick with simple, tamper-proof jars.
Cost always enters the conversation, but so does storage, safety, and shipping. Hazardous material regulations add another layer of hassle. I’ve seen labs chew through budgets on shipping alone, so keeping things small helps. Meanwhile, firms buying bulk might save up front but must follow strict rules. People often mistake this for mere bureaucracy, but it keeps shipments safe and prevents supply chain headaches.
Using higher purity in low-stakes manufacturing can feel like driving an F1 car in bumper-to-bumper traffic. Some processes accept impurities just fine, while others unravel if things aren’t close to perfect. This nuance gets lost in online shopping pages, but anyone working at the bench sees it with every new bottle.
The toughest spot comes for small operations stuck between research scale and full industrial scale. Ordering exactly what you need isn’t always straightforward. I've heard colleagues vent about minimum orders that dwarf their actual usage, and then they end up with waste or expired chemicals. Some suppliers now experiment with custom batch sizes, which feels like a step in the right direction.
Digital inventory management and direct-from-manufacturer models can bridge the gap between lab and plant floor. Broader awareness about safe disposal, proper storage, and labeling will help smaller buyers avoid costly mistakes. If vendors listen more to the needs of diverse users, everyone benefits—less waste for the lab, better margins for the distributor, safer workspaces all around.
| Names | |
| Preferred IUPAC name | 1,3-benzothiazol-2-amine |
| Other names |
2-Aminobenzothiazole 2-Benzothiazolamine Benzothiazol-2-ylamine 2-Benzothiazolylamine o-Aminobenzothiazole |
| Pronunciation | /tuː əˌmiːnoʊ ˌbɛnzoʊ θaɪˈæzoʊl/ |
| Identifiers | |
| CAS Number | 136-95-8 |
| Beilstein Reference | 120986 |
| ChEBI | CHEBI:18218 |
| ChEMBL | CHEMBL562 |
| ChemSpider | 12253 |
| DrugBank | DB08198 |
| ECHA InfoCard | InChIKey=WMWWEQIUJRGUOY-UHFFFAOYSA-N |
| EC Number | 202-729-7 |
| Gmelin Reference | 5298 |
| KEGG | C06568 |
| MeSH | D000572 |
| PubChem CID | 7001 |
| RTECS number | CV8400000 |
| UNII | 8HUM3L8B7T |
| UN number | UN2583 |
| Properties | |
| Chemical formula | C7H6N2S |
| Molar mass | 150.20 g/mol |
| Appearance | Light yellow to brown solid |
| Odor | odourless |
| Density | 1.36 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.46 |
| Vapor pressure | 0.00114 mmHg (25°C) |
| Acidity (pKa) | 10.2 |
| Basicity (pKb) | 9.3 |
| Magnetic susceptibility (χ) | -51.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.744 |
| Viscosity | 2.02 mPa·s (at 80 °C) |
| Dipole moment | 3.97 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 96.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 129.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3761 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause an allergic skin reaction. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 162°C |
| Autoignition temperature | 540°C |
| Lethal dose or concentration | LD50 (oral, rat): 750 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 750 mg/kg |
| NIOSH | RN0150000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-Aminobenzothiazole: Not established |
| REL (Recommended) | 100 mg |
| Related compounds | |
| Related compounds |
Benzothiazole 3-Aminobenzothiazole 2-Mercaptobenzothiazole 2-Chlorobenzothiazole 2-Methylbenzothiazole |