Long before most of us had ever heard the name, chemists working in the early 1900s discovered benzothiazole-2-thiol as they began stitching together new sulfur-nitrogen compounds. The labs of Friedrich Bayer and colleagues in Germany buzzed with experiments trying to unlock new rubber additives, dyes, and pharmaceuticals. Over decades, researchers tweaked the original Benzothiazole framework, adding or substituting atoms, until a sulfur atom landed at the two position, linking the molecule’s aromatic ring with a sulfur handle that gave it unique reactivity. The molecule’s wide adoption in the rubber industry exploded after laboratories published results showing sharp improvements in vulcanization and anti-degradation. Chemists, myself included, often marvel at how a simple alteration—shifting a single atom—can launch entirely new industries and fields of research.
Benzothiazole-2-thiol often gets its start as a yellowish crystalline powder, sometimes forming plates or needles, depending on the solvent used in crystal formation. Its molecular backbone features fused benzene and thiazole rings, with a thiol group (-SH) providing a reactive site. Today, product grades differ based on purity and trace contaminants, usually defined in parts per million for use in critical sectors like pharmaceuticals and advanced materials. My own work with rubber vulcanization exposed me to pharmaceutical-grade samples that cost exponentially more than their industrial cousins because drug manufacturers handpick them for their minimal impurity profiles. Each batch receives meticulous labeling denoting grade, recommended storage, and expiry, a must for regulatory and quality control audits.
On a lab bench, Benzothiazole-2-thiol stands out for its fairly high melting point—normally around 155–157°C—making it stable in processes where heat plays a role. Its distinctive odor, sometimes pungent, signals the free sulfur atom, something chemists learn to associate with reactivity and, occasionally, toxicity. The molecule dissolves well in ethanol, ether, dilute alkali, and organic solvents but resists water, forming only negligible solutions. This behavior matters during formulation; for example, rubber and polymer engineers use organic solvents to blend it into masterbatches, knowing water won’t break it down during storage or transport. Its thiol group, ready to bond with heavy metals or as a nucleophile in chemical syntheses, transforms Benzothiazole-2-thiol from a lab oddity into a core reagent for both large-scale and fine chemical applications.
Manufacturers routinely track moisture content, melting point, purity, and the presence of heavy metals or other trace contaminants. Labels detail the chemical’s systematic and common names, batch number, manufacturing and expiration dates, recommended storage temperature, and standard safety warnings. Labels may also include hazard diamonds, UN numbers, and instructions for dealing with accidental releases—a requirement enforced by global chemical safety agreements. Proper certifications, like ISO standards for lab use and Good Manufacturing Practice (GMP) stamps for pharmaceutical applications, assure downstream users that each kilogram meets regulatory demands. Handlers—myself included—double-check labeling for discrepancies, since improper storage can render what should be a stable powder a hazardous mess or a completely inert failure.
Traditionally, chemists started with ortho-aminothiophenol and condensed it with carbon disulfide or similar sulfur-based agents under controlled heat. This process, refined over decades, usually took place in the absence of air to keep the highly reactive thiol stable. Modern industrial routes focus on maximizing yields and minimizing toxic byproducts, such as hydrogen sulfide, by using enclosed systems and precise stoichiometric controls. Scale-up, which I witnessed in chemical plants, brings its own set of challenges: reactions go exothermic, impurities crop up at parts-per-thousand rather than mere trace amounts, and large filtration setups are needed to capture final powder. Recent developments in green chemistry seek to cut down solvent waste and energy usage, but the classic methods still dominate.
The -SH group at the two position acts like a magnet for transition metals and alkylating agents. Synthetic chemists exploit this functionality, forming metal complexes with applications in analytical chemistry or transforming the group into sulfonates, ethers, or thioesters. Dehydrogenation can yield disulfide derivatives, which may carry different biological or industrial functions. Some researchers attach alkyl chains or aromatic substituents to change how the molecule interacts with biological enzymes or polymer matrices. In my own graduate research, swapping out the thiol hydrogen for a methyl group unlocked a series of reactions that built new, photoreactive materials for imaging. Each tweak throws open another door, whether it’s in catalysis, dye creation, or crop protection.
Depending on the application, Benzothiazole-2-thiol goes by several names. The International Union of Pure and Applied Chemistry (IUPAC) designates it as 1,3-benzothiazole-2-thiol. Old textbooks and catalogues reference its other guises: "2-Mercaptobenzothiazole" or its commercial shorthands like MBT, Vulkacit, or Captax, especially in the context of rubber and elastomer chemistry. Watch for confusion—rubber additives often show up in safety data sheets and shipping manifests under trade names rather than chemical identifiers, a point I stress to new lab techs starting in rubber compounding. Regulatory agencies prefer the full name when listing hazardous substances and workplace exposure limits.
Benzothiazole-2-thiol needs respect in any setting. Inhalation, skin contact, or ingestion can lead to dermatitis, respiratory distress, or, in rare cases, more serious systemic effects. Facilities enforce fume hood use, nitrile gloves, and—where bulk handling occurs—full-body personal protective equipment (PPE). Occupational exposure limits vary but generally fall below one milligram per cubic meter in air. In storage, containers require tight seals and segregation from oxidizers or strong acids, as these can spark unintended reactions. Engineers design local exhaust ventilation and spill response plans so workers have clear steps in case of accidental release. Training focuses on everything from recognizing exposure symptoms to proper clean-up of powder spills, given the compound’s stubborn ability to linger on surfaces.
In my experience, rubber curing dominates its industrial profile; tire makers and conveyor belt factories go through tons of Benzothiazole-2-thiol annually. It accelerates vulcanization, binding sulfur atoms into long polymer chains, but also works as a corrosion inhibitor for metals, a key intermediate for dyes, and a building block in certain antifungal and antibacterial agents. Pharmaceutical researchers tap it for scaffolds during the synthesis of anti-inflammatory or anticancer candidates. Electroplaters use it to keep copper and brass surfaces gleaming. Each industry pushes for specialist grades, from ultrapure for health applications to robust, cheaper grades for mass-market rubber processing.
The labs I’ve worked in partner with universities and companies looking to build on Benzothiazole-2-thiol’s scaffolding. Recent patents highlight hybrid catalysts, light-activated photoinitiators for 3D printing, and medicinal chemists’ quests for better biological assays. Material scientists focus on surface-modified polymers that leverage the thiol’s affinity for metals, hoping to create sensors and efficient water filters. Environmental chemists try to predict and curb any long-term breakdown products leaching from plastics or anti-corrosion coatings. As the need for sustainable chemicals grows, researchers invest in greener synthesis routes and scrutinize every downstream degradation pathway. Real breakthroughs often come from outside the original field, a reminder of how interconnected chemical innovation remains.
Toxicologists track acute and chronic effects in animal models and occupational cohorts. Benzothiazole-2-thiol can sensitize the skin and sometimes acts as a weak mutagen. Ecotoxicologists have measured its persistence in soil and water runoff near tire manufacturing zones. Regulatory agencies such as the European Chemicals Agency ask companies to submit detailed dossiers outlining human and environmental risks before approving expanded use. The molecule’s breakdown products occasionally trigger persistence and bioaccumulation flags, so researchers track environmental concentrations and toxic endpoints in affected species. Practical efforts to minimize exposure include containment, substitution with safer alternatives, and constant revision of exposure limits based on the most up-to-date findings.
The way forward centers on cleaner production, stronger toxicity controls, and extending Benzothiazole-2-thiol’s application reach. Chemists see potential in recyclable functional polymers, new classes of anti-corrosion coatings for green infrastructure, and as a platform for advanced pharmaceuticals embodying both high activity and low side effect profiles. Industry researchers rethink old rubber vulcanization formulas to work under milder, energy-saving conditions. Environmental advocates look for degradable analogs, monitoring how these alternatives fare in real-world ecosystems. Academic partnerships will likely continue feeding cross-disciplinary advances, blending materials science, environmental chemistry, and molecular biology. As more regulations tighten, the compound’s complex history intersects with fresh questions about chemical stewardship and sustainable manufacturing.
Benzothiazole-2-thiol isn’t a name that comes up in everyday conversation, but its impact reaches far beyond the laboratory. Over time, I’ve come to appreciate the importance of such compounds by noticing how they pop up in unexpected corners – from the tires on my car to the sunscreen that keeps skin safe. Companies rely on benzothiazole-2-thiol for its power to speed up vulcanization, that chemical trick which gives rubber its strength and elasticity. Without this process, tires would wear out faster and hoses would crack. The safety gear firefighters trust and the boots workers wear on construction sites both owe part of their resilience to compounds like benzothiazole-2-thiol.
Years ago, I learned about rubber compounding in a manufacturing plant. The process seemed simple at first—mixing chemicals, heating them up, pressing them into molds. Things changed the day I watched a batch of rubber fall apart because the right accelerator wasn’t added. That mistake taught me that choosing the right additives makes all the difference. Benzothiazole-2-thiol often earns its spot in the recipe because it’s consistently reliable and helps the material last. Science backs this up: studies link its use to better resistance to wear and improved performance under high temperatures.
No chemical comes without its baggage. The same traits that make benzothiazole-2-thiol useful in rubber can raise questions. Overuse or improper handling leads to leakage into water and soil. Research from environmental agencies shows that benzothiazole-related compounds can persist and break down slowly. Some scientists have raised concerns about potential toxicity for aquatic life and possible allergic reactions in sensitive people.
This is a reminder that progress and responsibility walk hand in hand. The answer isn’t simply to remove every useful chemical; the real challenge involves making sure they’re used wisely and managed safely. Leading tire and rubber manufacturers have begun to tighten up how they use and dispose of benzothiazole-2-thiol. Environmental groups encourage not just safer handling, but also regular monitoring of factory wastewater to keep harmful residues out of rivers. Personally, I’ve come to respect companies that publish transparent reports about their processes and invite third parties to audit them. It builds trust and helps keep them on track.
Most folks never think about the chemical science that keeps their shoes waterproof or their car safe on a rainy day. Greater awareness helps people ask questions and hold manufacturers to higher standards. For me, learning about benzothiazole-2-thiol’s journey from laboratory to finished product serves as a daily lesson: even the smallest ingredient makes a difference. By putting good science, ethics, and accountability together, industries can keep reaping the benefits while protecting communities and natural resources.
Benzothiazole-2-thiol belongs to a group of organosulfur compounds. You see a benzene ring fused to a thiazole ring in this structure. Neither part stands alone; they merge to give the molecule its character. The thiazole ring brings together sulfur and nitrogen. At the 2-position on the thiazole ring, a thiol group (–SH) attaches. This location matters, both for how the molecule reacts and how it interacts in practical settings.
Put it all together: You end up with the chemical formula C7H5NS2. Picture this backbone: a hexagonal benzene, a five-membered ring with both nitrogen and sulfur fused at the side, then a single thiol group hanging off position number two. Chemists often draw it with the molecular shorthand: the benzothiazole fused system, with a –SH group at location two.
Even from my college days, learning about molecules like this showed me how small changes in a ring system can shift the whole game. That thiol group makes benzothiazole-2-thiol more reactive than its sulfide cousin. The placement of sulfur and nitrogen impacts not just chemistry on paper but real-world outcomes in research and manufacturing.
Industries never pick a molecule by accident. They use benzothiazole-2-thiol in rubber processing as an accelerator. The structure lets it form crosslinks faster, which boosts vulcanization. Research points out that sulfur atoms enhance reactivity with a variety of substrates, and the aromatic core allows stability without taking away flexibility.
Looking at the structure, one can guess certain risks. The thiol group stands out. Compounds with this group often carry an odor and tend to react with air, producing sulfonic acids. Reports show benzothiazole-2-thiol needs careful handling—skin contact, inhaling dust, or spills can have effects. As someone who’s spent time in labs, even the smallest crystals left out will start to darken, a sign of oxidation. Proper gloves, fume hoods, and storage avoid exposure and accidents.
Environmental scientists have flagged derivatives of benzothiazole as persistent. Its fused ring system doesn’t break down quickly in the wild. Runoff from rubber plants or chemical production can introduce these molecules into water sources. Recent papers from environmental chemistry journals stress how these molecules stick around in streams and lakes, affecting aquatic life.
Alternative accelerators exist, but the structure of benzothiazole-2-thiol still delivers strong performance for industry. Cleaner processes focus on limiting exposure and improving containment in wastewater. Years ago, global initiatives for “greener chemistry” drew attention to replacing problematic chemicals. Some companies now employ closed-loop systems to capture and treat waste. Bench-level research looks for ring modifications—either blocking breakdown-prone spots or swapping in less persistent atoms—but any change demands testing for both safety and utility.
The chemistry behind benzothiazole-2-thiol does more than fill a line in a textbook. Its real-world use carries weight for production, safety measures, and environmental impact. The unique chemical structure shapes all these outcomes, for better or for worse, so deep understanding pays off—whether you’re in the lab, the boardroom, or hands-on with the materials.
Handling chemicals might look routine for some folks, but Benzothiazole-2-Thiol calls for a sharper focus than many. Anyone who's spent time with sulfide compounds can tell you, the smell alone tries to warn you away. But this compound brings more than a harsh odor. Benzothiazole-2-Thiol can easily irritate your skin, eyes, and lungs—the stuff that sends you home early with a rash or worse, a trip to urgent care. Long-term mishandling could mean chronic skin diseases or respiratory issues, which no lab coat or gloves will fix after the fact. Years in the lab have made me respect these risks more than any textbook.
Gloves aren’t an optional accessory. Nitrile or neoprene gloves hold up much better than cheap latex when Benzothiazole-2-Thiol shows up on your bench. Eyes need serious coverage, so safety goggles must sit snugly, not perched on your head like sunglasses. Even a single splash could burn or blind. Laboratory coats need to close tightly at the wrists and neck, and loose sleeves or gaps make easy entry points for fine powder. Breathing protection matters—fume hoods swallow up vapors before they hit your nose and lungs. You shouldn’t rely on basic masks with this kind of compound. Respirators rated for hazardous particulates and organic vapors become essential if spills escape containment.
Mistakes always find their way into a lab, so planning for spills matters just as much as steady hands. Once, two grams slipped from a dish onto the bench and cascaded right toward my pack of samples. Old habits saved me that day—spill pads came out fast, along with plenty of soapy water and a scoop for the powder. Cat litter or sand can do in a pinch, trapping the chemical before it escapes on your shoes. Disposing that waste means treating it like the hazardous stuff it is—sealed, labeled, and nowhere near the regular trash. Chemical hygiene starts with not trusting the person before you to clean up your mess.
Even the best-run laboratory suffers from fumes, especially with organosulfur chemicals. Fume hoods need to actually work, not just hum to themselves. Test their airflow with a piece of tissue or a gentle exhalation. Good air movement cuts down the indoor air contamination that can leave everyone feeling sick by the day’s end. Keep Benzothiazole-2-Thiol stored in dry, cool conditions, out of direct sunlight or heat. Glass or compatible plastic containers, tightly sealed, help prevent leaks and slow down degradation. Storing acids and bases away from this compound avoids dangerous reactions, since mixing these by accident creates new hazards nobody wants to clean up.
A thick binder of safety rules won’t protect anyone if the team skips training. Every new lab worker deserves hands-on instruction for chemicals like Benzothiazole-2-Thiol. Teach them to recognize the signs of exposure—red skin, cough, headaches—and to treat every drop as something worth worrying over. Emergency showers and eyewash stations should stay clear of clutter and actually work—test them yourself, not just during annual checks. Supervisors need to support time away from the bench to review Material Safety Data Sheets together.
Nobody handles Benzothiazole-2-Thiol in a vacuum. Sharing best practices across labs helps raise the bar for chemical safety. Reporting accidents—even minor spills—lets others learn and prevent bigger disasters. Universities and workplaces should run regular safety refreshers, not only for newcomers but for old hands who think they know it all. Investing in modern ventilation, real chemical storage cabinets, and actual emergency drills prevents the costliest mistakes. Safety isn’t only a set of rules; it’s a habit built from real experience, daily vigilance, and respect for every step in the chemical handling process.
Every compound has a story behind its numbers. For Benzothiazole-2-Thiol, that number—167.25 g/mol—is more than a figure at the bottom of a data sheet. This number represents years of research, days in the lab, and sometimes, a single experiment’s line between right and wrong results. I’ve stood in a lab trying to weigh out just the right amount of this material, hoping the calculation matches up under pressure. Getting the molecular weight right means fewer mistakes, less wasted material, and a step closer to results that make sense.
Benzothiazole-2-Thiol draws serious attention in research circles and chemical plants. Chemists use it to craft rubber accelerators, dyes, and specialty pharmaceuticals. Messing up a compound's molecular weight—whether it’s Benzothiazole-2-Thiol or something less exotic—can throw off yields, muddy product purity, and even trigger safety risks. Picture a reaction where you think you have 100 grams, but you're off by just a few grams due to a wrong weight. In a scaled-up process, that’s a disaster waiting to happen. Experience shows the margin for error shrinks fast as the scale grows.
From fact sheets to regulatory paperwork, precise numbers build trust. Researchers rely on established sources for values like 167.25 g/mol. The PubChem Database and Reaxys provide verifiable confirmation. Getting verification from these sources matters, especially after recent stories about database hacks and the dangers of relying on crowd-sourced chemistry details. It’s a reminder that "close enough" isn’t safe, especially when people’s health, money, and reputations are on the line.
For those working in environmental science or health, Benzothiazole-2-Thiol isn’t just a lab curiosity. It shows up as an industrial contaminant, found in river water near manufacturing sites, or even in household products. Proper risk assessment starts with knowing exact figures. Regulators setting safety limits for water pollution must have the right molecular weights for their calculations to mean anything. Otherwise, those permitted levels can’t protect either nature or people.
The same precision carries through clinical and pharmaceutical research. Dosages, toxicity studies, and quality assurance protocols all hang on the proper weight. If you’ve ever seen the scramble in a quality control lab when a single figure doesn’t add up, you know why accuracy matters. Pharma companies pour millions into drug development. One slip—rooted in something as basic as molecular weight—can send a whole project up in smoke, along with investor trust.
Relying solely on a single, outdated reference costs more than you’d expect. Anyone handling Benzothiazole-2-Thiol should double-check numbers through trusted databases and current scientific literature. Teaching lab newcomers about this practice saves headaches later, turning good habits into a culture of accuracy. Electronic lab notebooks and digital reference tools help close the gap. Regular training and updates—for everyone from entry-level techs to senior chemists—are always worth the time investment.
It’s tempting to treat molecular weight as a detail, but ignoring accuracy here is the fastest way to run into setbacks. That 167.25 g/mol figure doesn’t just solve an equation. It protects people, budgets, and the integrity of the work. For anyone who spends their days with chemicals, those reasons hit close to home.
Benzothiazole-2-thiol, also known in labs as 2-mercaptobenzothiazole, has a reputation for both utility and caution. It plays a big role as a rubber accelerator and pops up in pharmaceuticals and corrosion inhibitors. Being around chemicals for years teaches a person that common sense saves headaches in the long run, and that’s especially true here. This compound brings both value and risk: inhalation and skin contact can trigger reactions ranging from irritation to more serious symptoms. So, the place where this stuff gets stored and who has access becomes more than just a box to check off—it’s a basic step for safety and quality.
A dry, well-ventilated space always beats a corner of a damp basement for storage of any chemical, but Benzothiazole-2-thiol makes the point clear. Moisture hangs in the air in some labs and warehouses, and this compound doesn’t forgive exposure to humidity. Contact with water or even just humid air can lead to slow decomposition. Storing it in airtight containers, preferably glass or high-grade plastic, blocks that threat. Desiccators lined with silica gel go a step further for those in damp climates.
Shelf life shrinks with sunlight and high heat. I’ve seen temperature swings warp labels and caps; with Benzothiazole-2-thiol, heat actually speeds up degradation. Room temperature storage supports stability, and keeping it out of direct sunlight stops UV breakdown. This isn’t about overkill—photodegradation strips away the effectiveness of chemicals and can introduce new hazards. A solider, dark cabinet in a temperature-controlled room does the trick. Heat sources like radiators and steam pipes should be kept away from storage shelves, and refrigeration isn’t usually called for unless noted by the supplier’s safety sheets.
Keeping chemicals organized by compatibility is a lesson learned from too many minor spills. Benzothiazole-2-thiol belongs nowhere near oxidizers and acids. Strong oxidizers can ignite, and acids trigger reactions that fill the room with sulfur compounds—a stink few forget. Secure labeling with clear hazard markings makes life easier for those coming after you, cutting confusion and slip-ups.
The best storage isn’t just physical. People handling this compound ought to have access to material safety data sheets at all times. These are more than paperwork—they are lifelines when something spills or exposure happens. Gloves, goggles, and decent ventilation reduce the chance of exposure, keeping workers and students out of harm’s way. Spill kits tailored for sulfur compounds near the storage site offer peace of mind. Regular inventory, checking for container leaks and discoloration, stops surprises.
Tight budgets and crowded shelves often squeeze out safety. Old habits, like reusing containers or stashing bottles where there’s space, put everyone at risk. Good training helps, but what actually changes behavior is routine checks and simple systems—a good storage log, time-stamped inspections, and reminders about what can go wrong. Digital inventory tracking reduces guesswork about what sits on the shelf too long, and clear accountability means mistakes get caught quickly.
Better public awareness, more consistent labeling, and smarter purchasing policies would go a long way in improving chemical safety for Benzothiazole-2-thiol and its cousins. At the end of the day, treating this compound with the respect earned through real-world experience and evidence not only protects health and safety but also extends the working life of valuable materials.
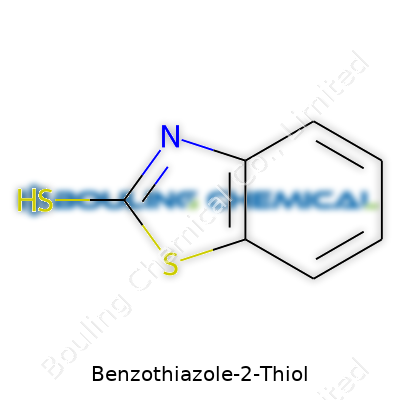
| Names | |
| Preferred IUPAC name | 1,3-benzothiazole-2-thione |
| Other names |
2-Mercaptobenzothiazole MBT Mercaptobenzothiazole Benzothiazole-2-thione 2-Benzothiazolethiol |
| Pronunciation | /ˌbɛnzoʊˌθaɪəˈzoʊl tuː ˈθaɪɒl/ |
| Identifiers | |
| CAS Number | 149-30-4 |
| Beilstein Reference | 120825 |
| ChEBI | CHEBI:18137 |
| ChEMBL | CHEMBL18703 |
| ChemSpider | 211057 |
| DrugBank | DB14628 |
| ECHA InfoCard | 03a0c8be-e094-4a0d-9622-ee0937a0c2a5 |
| EC Number | 3.1.6.20 |
| Gmelin Reference | 7923 |
| KEGG | C01890 |
| MeSH | D001578 |
| PubChem CID | 6977 |
| RTECS number | DN0175000 |
| UNII | 1L2B9DT92M |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C7H5NS2 |
| Molar mass | 167.24 g/mol |
| Appearance | Light yellow crystalline powder |
| Odor | Characteristic; unpleasant |
| Density | 1.42 g/cm3 |
| Solubility in water | slightly soluble |
| log P | 1.67 |
| Vapor pressure | 0.000661 mmHg at 25°C |
| Acidity (pKa) | 7.02 |
| Basicity (pKb) | 4.37 |
| Magnetic susceptibility (χ) | -54.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.730 |
| Viscosity | 3.83 mPa·s (at 160 °C) |
| Dipole moment | 2.97 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 117.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -70.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -301 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | D08AX05 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation, may cause an allergic skin reaction. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | CC1=CC=NC2=CSC2=C1 |
| Signal word | Warning |
| Hazard statements | H301, H311, H331, H315, H319, H335 |
| Precautionary statements | P261, P273, P280, P302+P352, P305+P351+P338, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 1, Instability: 0, Special: -- |
| Flash point | 162 °C |
| Autoignition temperature | 450 °C |
| Lethal dose or concentration | LD50 oral rat 2630 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 1500 mg/kg |
| NIOSH | SN2975000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Benzothiazole-2-Thiol: 0.1 mg/m3 |
| REL (Recommended) | 0.3 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
2-Mercaptobenzothiazole Benzothiazole Benzothiazole-2-sulfonic acid 2-Aminobenzothiazole 2-Benzothiazolamine |