Every time I dig into a compound like benzomorpholine, I end up tracing a winding path that stretches back to the early days of modern organic chemistry. In the twentieth century, research communities set their sights on nitrogen-containing heterocycles. Benzomorpholine appeared as an odd but interesting cousin to the more famous morpholine family. Early chemical catalogs listed it among peculiar building blocks slightly overshadowed by more commercially prominent structures. Laboratories in Europe and the United States both experimented with ring-closure techniques to create the benzomorpholine core. Journals from the 1970s hold stories about both its unpredictable reactivity and its usefulness as a precursor in drug design, back when chemists mostly cared about morphine derivatives but occasionally took detours for molecules like this one. Its gradual rise in pharma libraries hints at a history shaped by serendipity as much as by deliberate innovation.
A bottle of benzomorpholine doesn’t attract much attention on the shelf. It’s just a colorless or slightly yellowish liquid, and can easily get lost among the similar-shaped bottles lined up in the reagent cabinet. Chemists interested in new scaffolds for medicinal chemistry eventually come across it, especially those who chase after new CNS-active compounds or novel enzyme inhibitors. My colleagues from medicinal chemistry refer to it as a “Swiss army knife” because its structure fits plenty of molecular designs without taking up much space. It tends to show up as an intermediate, or as a molecular fragment that lands in bigger, more complex molecules designed for biological activity studies.
Picking up benzomorpholine means dealing with a compound whose boiling point averages between 245-255°C, and a melting point low enough that it usually appears as a liquid at room temperature. On a molecular level, it features a fused aromatic ring and a morpholine ring, which gives it a hybrid of aromatic stability and the unpredictable reactivity of nitrogen heterocycles. Its molecular formula, C8H9NO, makes it relatively light but not very volatile. With a moderate polarity, it dissolves well in most common organic solvents; water solubility stays limited given the aromatic component. From experience, chemists treat it like similar morpholine derivatives, knowing that the benzene ring throws in some extra complications during synthesis or modification.
Vendors labeling benzomorpholine usually reference the CAS number (2523-07-7), with purity standards hovering above 97% for research-grade material. I’ve found technical data sheets listing refractive index near 1.570, density slightly above 1.1 g/cm³, and basic hazard data rooted in its amine content. The packaging usually carries warnings about harmful vapor exposure and includes provisions for proper storage in cool, well-ventilated spaces, sheltered from light and oxidizing agents. Safety Data Sheets (SDS) repeat standard warnings for nitrogen heterocycles—organ irritation, potential neurotoxicity, and a need for gloves and goggles during handling. Labeling for export or industrial procurement often flags it as a precursor with potential dual use, especially in countries with strict chemical controls.
In practice, benzomorpholine synthesis doesn’t call for exotic hardware. Most routes start with o-aminophenol and ethylene oxide or chloroethanol, relying on straightforward nucleophilic substitution or cyclization reactions. Many chemists favor base-catalyzed conditions, using potassium carbonate in a solvent like DMF to get cyclization rolling. Breathing in the smell of amines always signals the start of reaction progress, and by the time the reflux kicks off, the mixture shifts color slightly as the ring closes. Some labs push the reaction with microwave heating for faster yields. Purification usually involves distillation or column chromatography. The yield depends on vigilance—impurities crop up unless reactants stay pure and reaction times get tightly controlled.
Benzomorpholine stands out for what it does after its creation. It handles substitution at the aromatic ring, where electrophilic reagents like bromine add new handles for further synthesis. The nitrogen also gets targeted for alkylation or acylation to diversify the core. In the hands of a skilled chemist, the molecule turns into a launching pad for building blocks tailored toward biologically active compounds or ligands for metal complexes. I’ve watched teams run hydrogenation, halogenation, and oxidation, each with their quirks, but most follow predictable morpholine chemistry. Its durability under mild reaction conditions means it survives the early steps of multi-stage synthesis with less fuss than similar compounds—an asset to anyone stitching together new molecules in the lab.
Conversations about benzomorpholine often get tangled up by the variety of names on the bottle. Besides “1-benzomorpholine,” you’ll find “Benzo[b][1,4]oxazinane,” “o-phenyloxypiperidine,” and numerous registry numbers, depending on which supplier or publication you read. Some catalogs opt for “2,3-dihydro-1,4-benzoxazine” to reflect subtle structural differences or ring closures, even if it points to close relatives. Any chemist working across international borders soon learns to double-check structures, not just names, since inconsistency in labels can lead to order mishaps and research delays.
Handling benzomorpholine means taking personal protective equipment seriously. Studies suggest inhalation or prolonged skin contact risks organ irritation, so lab coats, gloves, and eye protection stay non-negotiable. Fume hoods aren’t merely for comfort, as even trace amine vapors accumulate and make the atmosphere unpleasant or unsafe over time. Emergency protocols cover accidental spills and discuss quick use of absorbent materials, plus detailed logbooks to track who handled what and when. Laboratories storing benzomorpholine monitor inventory closely since regulatory frameworks in places like the United States and Europe require precise records to prevent unauthorized use or loss. Collecting waste comes with its own rules, never mixing benzomorpholine residues with oxidizers or acids to avoid unpredictable reactions.
Benzomorpholine threads itself into pharmaceutical research, especially where medicinal chemists hunt for new scaffolds with CNS activity. Some early antipsychotic or antidepressant candidates included benzomorpholine derivatives, though few reached the clinic. The molecule appears in synthetic routes for dyes, agrochemicals, and catalysts, offering a compact but modifiable building block. In drug discovery, libraries built from benzomorpholine core structures fill out screening plates for G-protein coupled receptor assays or kinase inhibition panels. Chemists in material science sometimes tap its structure in organic electronics or polymer research, aiming to tweak electronic properties through subtle molecular changes. These application areas rarely unfold in isolation; teams pull benzomorpholine into different projects as soon as a need arises for a stable, reactive, and compact nitrogen heterocycle.
Screening the latest literature, I notice research clusters focused on enhancing benzomorpholine’s synthetic accessibility and reactivity. Some groups work to streamline routes from renewable or safer feedstocks, aiming to cut hazardous byproducts. Ongoing collaborative projects assess how tweaks to the core structure boost selectivity or potency in drug targets. Automation and high-throughput screening drive new discoveries, sending hundreds of benzomorpholine-derived fragments into biological screens overnight. The compound’s electronic flexibility catches attention from researchers in coordination chemistry, who use modified derivatives to control metal-ligand binding for catalytic applications. Funding agencies in Asia and Europe seem receptive to grant proposals centered around new benzomorpholine analogues, especially if they tick boxes for sustainability, economic synthesis, or disease relevance.
Lab safety officers rarely let researchers forget the need for thorough toxicity studies. Early reports suggest benzomorpholine stays less toxic to mammalian cells than many open-chain amines, yet chronic exposure data remains patchy. Regulatory frameworks urge review of acute inhalation, ingestion, and dermal contact effects. Animal studies rarely mention severe systemic effects, but researchers in environmental chemistry worry about slow breakdown and potential bioaccumulation. Some studies point out the need to examine long-term neurotoxicity, especially since nitrogen heterocycles crop up in environmental pollutants with subtle health impacts. Safety committees in both academia and industry insist on gradual scale-ups before trying benzomorpholine at pilot or industrial scale, demanding updated risk assessments with every new application or formulation.
Looking ahead, new synthetic strategies seem poised to make benzomorpholine and its analogues even more accessible for researchers. Automated chemistry, AI-driven retrosynthesis, and green chemistry—the usual buzzwords—shape the direction of innovation. The compound’s core structure leaves plenty of room for modifications aimed at targeting emerging biological pathways, designing next-generation materials, or developing environmentally friendly agrochemicals. Tech companies interested in biosensors or smart coatings occasionally reach out to the academic community for ideas using benzomorpholine’s unique reactivity. The push for sustainability puts pressure on chemists to design cleaner, safer, and more cost-effective production methods. Not every project leads to a breakthrough, but the steady trickle of interest in benzomorpholine—from pharma to material science—signals that its story is nowhere near finished.
Benzomorpholine doesn’t top the news or turn up in daily conversations, yet this chemical touches more parts of modern life than most realize. I picture a world without some of these “background” chemicals and it gets messier pretty fast—medicines become less effective, and certain industrial processes stall.
Pharmaceutical researchers lean on benzomorpholine as a building block. Chemists tweak the core structure to design new medicines, especially those targeting the central nervous system. The reason is tied to its unique ring structure—imagine it as a versatile LEGO piece that can attach in several spots.
Take a look inside the lab: drug designers rely on benzomorpholine to add stability or tweak how a medicine interacts with the body. Some studies show that drugs containing a benzomorpholine backbone stick around longer in the bloodstream, often letting patients take fewer doses. From what I’ve seen, these compounds sometimes pop up as key parts in drugs meant for mental health and pain management.
Outside medicine, this chemical gets pulled into action by those developing new pesticides or advanced plastics. Crop scientists use it in efforts to build safer pest solutions—safer for people, tougher on insects, and less likely to poison groundwater. Even in plastics and dyes, benzomorpholine can boost thermal stability, which comes in handy for materials that have to endure high heat.
Industrial chemists see it as a shortcut that helps shave time off multistep syntheses. I’ve met a few who use it to anchor tricky chemical reactions—they swear it keeps production lines running smoother.
No magic wand here: every potent tool comes with trade-offs. Lab workers dealing with benzomorpholine need proper gear because breathing it in or letting it touch skin can lead to irritation. Regulations rightfully urge that companies keep tight controls on waste and exposure. At community scale, responsible disposal matters, since chemicals like these can run off or leach into water.
My own experience suggests that many in the chemical industry are not content just meeting minimum safety rules. Engineers and managers look for ways to keep workplace accidents and spills at zero. This can mean better training or improved ventilation, not just protective gloves.
Research teams keep searching for newer molecules that do the same jobs but break down faster in nature or have fewer health risks. Green chemistry groups have made some progress finding substitutes that get the job done with less environmental baggage. These changes don’t come overnight—testing and scaling up safe replacements costs money and time.
For anyone outside the lab, benzomorpholine stays out of sight. Yet what happens in factory backrooms and research centers does impact daily life, whether that means more reliable medication, safer foods, or fewer toxic leaks. Paying attention to “minor” chemicals once in a while reminds us that progress often depends less on the headline-grabbers and more on the quiet workhorses behind the scenes.
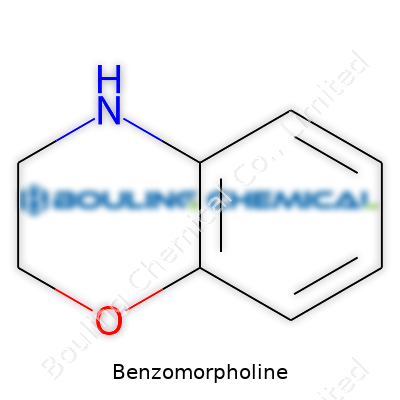
Benzomorpholine belongs to a group of compounds that carry a ring structure you might spot in a few different places, from research chemistry to specialty pharmaceuticals. At its base, the molecule combines a benzene ring with something called a morpholine ring. The morpholine part looks a bit like a six-membered circle, with oxygen and nitrogen right inside the ring alongside four carbon atoms. Put that with the familiar six-carbon ring of benzene, and the picture starts to form.
Spent enough time looking at chemical diagrams, you start recognizing patterns. I notice how fusing a benzene ring to a morpholine backbone shapes new possibilities for reactivity. The official chemical name—2,3,4,5-tetrahydro-1-benzoxazine—spells out just how the fusion sits. Chemists often depict it as a single continuous ring, with two arms (the oxygen and nitrogen) poking out like gateposts. That structure, even in a plain drawing, says a lot about how it might react with other chemicals or break down in the body.
The big story with benzomorpholine is all about what can hang off the base structure. Tiny tweaks on the ring, moving a hydrogen here or adding a methyl group there, can flip the whole behavior of the compound. A lot of solid research work turns on swapping out pieces on ring systems just like this. I’ve talked with folks in pharma labs who chase specific ring patterns all year, searching for a new lead or pathway.
Structure tells a chemist which compounds dissolve in water, which ones slip through membranes, or how tightly two rings push against each other. Just the shift from a carbon to a nitrogen atom in the ring flips polarity and influences binding to other molecules—sometimes in ways you only really see after months of testing.
Some molecules leave a wider footprint than others. Benzomorpholine and its cousins, because of the morpholine ring, offer a starting point for building antibiotics or exploring neurological drugs. Chemists use the core as a starting block, adding and swapping pieces until the results show promise. I've come across morpholine rings in antifungal agents, pesticides, and even specialty dyes. It’s not that the base structure does all the work, but it makes everything that comes after possible.
One thing that comes up in research meetings is how core structures with both nitrogen and oxygen allow for unique hydrogen-bonding opportunities. In English: it sticks or slides in different settings, opening up functions some other rings can’t perform. Sometimes, finding that one base structure with the right arrangement saves years of trial and error on a new drug or treatment.
Working with benzomorpholine, safety means everything. The presence of the nitrogen and oxygen centers, plus the aromatic ring, hints at possible reactivity. In the lab, storage and handling get strict—keep it dry, away from acids or oxidizers. Chemists spend a lot of effort crafting synthetic routes that use greener solvents, cut down on stages, and avoid harsh reagents. Some teams push to develop methods that pull in more of the starting materials, leaving less waste behind.
People in research, manufacturing, and regulation can keep up progress by sharing improved synthesis steps, pooling toxicity data, and updating safety sheets. The lessons from building and studying benzomorpholine give insights that ripple through the whole field of drug development and industrial chemistry. Tuning the chemical structure makes or breaks all kinds of innovation, and benzomorpholine rings sit right in the thick of it.
Anyone poking around for “Benzomorpholine” will probably find more questions than clear answers. Forgive the chemistry jargon, but Benzomorpholine isn’t the kind of name you hear in everyday talk. Its presence in chemical supplier catalogs comes down to two things: scientific demand and regulation. This isn’t bleach or baking soda, and the average pharmacy won’t stock it.
Diving into online chemical markets shows that you can run into a wall pretty fast. Chemical suppliers ask for company names, research intent, and plenty of documentation. If you’re not part of a registered organization or university lab, buying Benzomorpholine looks more like an uphill climb than a transaction.
The caution isn’t random. Benzomorpholine links back to research on psychoactive substances, drug design, and certain industrial uses. Regulators block the road precisely because of these connections. Concerns about misuse or dangerous byproducts fuel the strict rules. It’s a bit like buying a bag of fertilizer—if you want twenty tons, expect authorities to ask “what for?”
Anyone who’s taken an interest in Benzomorpholine probably cares about more than theory. The substance pops up in medicinal chemistry papers for its scaffold—chemists use that term to describe the core structure around which drugs may be built. Researchers test these scaffolds for a wide range of properties, hoping to discover something with real medical value.
The flip side: anything with room for chemical tweaking can spark the curiosity of less benign actors. History shows how seemingly harmless compounds can end up in the wrong hands, often tweaked into substances that wind up banned a decade later. Authorities remember what happened with synthetic cannabinoids and fentanyl analogs—no government wants to be caught off guard.
For legitimate scientists, the tight rules mean extra paperwork. Delays or denials in ordering get in the way of research. This isn’t just a headache. Delays can keep labs from developing potential cancer drugs or tools for brain research. If you’ve ever scrambled to finish an experiment while waiting for a shipment, you know how frustrating this can get. Medical progress sometimes moves at the speed of government paperwork.
For the public, the stakes run in two directions. On one side, tight restrictions may slow down valuable discoveries. On the other, loose rules risk dangerous new substances slipping out through loopholes. Any story about Benzomorpholine, or any obscure chemical, sits right at the intersection of cautious regulation and the drive for scientific discovery.
Scientists and policymakers keep looking for that sweet spot—a world where useful research isn’t stalled by red tape, but gates stay locked against misuse. Some countries use scientific advisory boards to review requests. Others lean heavily on transparent supply chains—knowing where chemicals come from and where they’re going. Digital record-keeping and closer monitoring offer ways to flag suspicious orders before harm spreads.
Anyone curious about Benzomorpholine should be ready to show credentials, fill out forms, and navigate rules. This field always demands trade-offs, and the call for fresh ideas on regulation never really stops. Real breakthroughs depend on researchers, lawmakers, and industry working together, not just tick-box compliance or blind prohibition.
Benzomorpholine doesn’t roll off the tongue easily, yet plenty of chemists and researchers have met this chemical in the lab. Skipping all the jargon, it’s a compound that might show up in pharmaceutical labs and academic research. It smells faintly amine-like, and trust me, it’s not the kind of thing anyone should be splashing around carelessly. I remember the first time I uncapped a bottle—it wasn’t panic, but it kept me on my toes. There’s good reason for that.
The skin hates benzomorpholine. Even fleeting contact could leave you itching for hours, or worse—think redness, burning, sometimes even blisters. Eye exposure hurts even more, so always pick up those safety goggles and keep a bottle of eye rinse handy. Ordinary lab gloves do the trick if you don’t forget to change them right away after a spill or splash. During a long day, it’s easy to forget you’re wearing gloves—until you touch your phone, door handle, or your own face, and drag the risk along with you.
This isn’t a chemical to use in a closed room with no air movement. Benzomorpholine gives off vapors that grab onto your nasal passages and throat without warning. I’ve seen folks try to “get away with” working in a quiet corner; a few minutes later, they’re coughing or their head is pounding. A fume hood should always be running before you even crack open a bottle. If local regulations call for a respirator during heavier use, don’t gamble. Long-term exposure could lead to breathing problems—and the research on its chronic effects is far from complete.
Sure, some chemicals just want to be left alone in a cool spot. Benzomorpholine begs for more respect. Tight lids keep fumes where they belong. Clear labels are a must—scratched or faded writing could lead someone to grab the wrong bottle. Nobody in the lab will enjoy tracking down the source of a strange smell, only to find an open container stashed behind another reagent. Locking up toxic chemicals isn’t just a rule; it keeps wandering hands and stray accidents from adding extra drama to the workday.
Accidents don’t follow schedules. A beaker tips over, a pipette slips—suddenly there’s benzomorpholine on the bench or worse, on the floor. Speed counts. Scoop up what you can using inert material like sand or absorbent pads. No paper towels, since soaking and wringing only spreads the mess. Proper disposal means following the chemical waste protocols every lab hands out, but too often get buried under paperwork. Training helps—a quick demo on cleanup sticks better in my mind than any colorful poster ever did.
Most lab veterans will agree, safety means a lot more than donning a lab coat and goggles. Talk with co-workers, run through what-ifs, and keep real spill kits where everyone can see them. Staying ready, double-checking labels, and following the rules seem dull until something goes wrong. The people who remember to take every simple step every time keep both themselves and their team out of the doctor’s office.
Science keeps making new molecules, and sometimes it feels like the market races to find uses for them before anyone fully understands what they do to the body. Benzomorpholine sounds fancy, sure, but the first thing on anyone’s mind should be whether it’s actually safe. People ask about side effects and toxicity because they know stories of “cutting-edge” drugs and chemicals turning out to be riskier than anyone guessed.
If you poke through available scientific papers, Benzomorpholine doesn’t pop up often. Not like aspirin or caffeine, which have dozens of studies and huge track records. So trying to figure out its risks feels a little like reading a recipe without knowing what half the ingredients do. I remember looking into off-label compounds used in niche research—the info sometimes comes from smaller studies, maybe from the 1970s or 80s, and often still has big blank spaces where you’d expect details about human safety.
Here’s what stands out about Benzomorpholine so far: Most data comes from lab rodents. Some researchers checked basic toxicity and watched for strange behaviors. Once given in high doses, animals sometimes showed signs of nervous system trouble: shaky movements, agitation, weird reflexes. High doses can stress organs like the liver or kidneys. That signals caution, not a green light. Rats aren’t people and what happens in a cage doesn’t always match what hits a human bloodstream. Still, this points toward possible concerns, especially for anyone thinking about using such a compound outside strict lab settings.
Drugs and chemicals jump from the bench to the public way too fast sometimes. I’ve seen people get burned by new supplements that were rushed to market, only for later reports to show hidden dangers. It pays to slow down, read the fine print, and stay skeptical of big promises without big data. Safety studies should last longer than a few weeks and shouldn’t gloss over less obvious side effects. In the case of Benzomorpholine, short-term studies don’t cut it. Things like liver toxicity or slow nerve damage take time to show.
Some drugs get pulled years after they were approved because real-world use uncovers nasty surprises. Anyone claiming Benzomorpholine is totally safe is either guessing or hiding something. Without robust data, you can’t even know about possible allergic reactions, long-term neurological effects, or how it interacts with other chemicals. History shows trust gets burned by those who ignore toxicology, whether with drugs or industrial solvents.
Rolling out newer compounds responsibly means rigorous, independent testing. Not just with rodents, but full human studies that look at a range of doses, test for subtle side effects, and follow people for more than a couple of days. Regulators should ask for proof and keep looking for problems even after something is approved. Open databases that collect side effect reports from doctors and patients—like the FDA’s FAERS program—help catch problems before they spiral.
The lesson learned from past chemical safety fiascos: Don’t act on hype. Wait for solid evidence. Everyday people deserve real answers, not vague promises or cherry-picked facts. In the case of Benzomorpholine, a dash of skepticism—and a steady demand for more research—keeps everyone safer. No shortcut replaces careful science.
| Names | |
| Preferred IUPAC name | 2,3,4,5-tetrahydro-1-benzoxazine |
| Other names |
4H-1-Benzomorpholine |
| Pronunciation | /ˌbɛnzoʊˈmɔːrfəˌliːn/ |
| Identifiers | |
| CAS Number | 252-94-2 |
| 3D model (JSmol) | `3D Model (JSmol) string for Benzomorpholine:` ``` C1C2=CC=CC=C2NCO1 ``` This is the SMILES string, which you can use in JSmol or similar molecular viewers to visualize the 3D model. |
| Beilstein Reference | 136873 |
| ChEBI | CHEBI:138182 |
| ChEMBL | CHEMBL2349382 |
| ChemSpider | 153826 |
| DrugBank | DB15687 |
| ECHA InfoCard | 100.018.942 |
| EC Number | 3.1.1.328 |
| Gmelin Reference | 108607 |
| KEGG | C10637 |
| MeSH | D12610 |
| PubChem CID | 13949 |
| RTECS number | BO8925000 |
| UNII | 3577I6F296 |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C8H9NO |
| Molar mass | 149.19 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.16 g/cm³ |
| Solubility in water | Insoluble |
| log P | 1.33 |
| Vapor pressure | 0.09 mmHg (25°C) |
| Acidity (pKa) | 5.94 |
| Basicity (pKb) | 5.38 |
| Magnetic susceptibility (χ) | -64.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.613 |
| Dipole moment | 3.58 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 253.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -80.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4012 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | N07BB10 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, causes skin irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-3-1 |
| Flash point | 91.7 °C |
| Autoignition temperature | Autoignition temperature: 410°C |
| Explosive limits | Explosive limits: 1.1–6.4% |
| Lethal dose or concentration | LD50 (rat, oral): 640 mg/kg |
| LD50 (median dose) | LD50 (median dose): 97 mg/kg (intravenous, mouse) |
| NIOSH | QJ0525000 |
| PEL (Permissible) | PEL for Benzomorpholine: Not established |
| REL (Recommended) | 0.6 mg/L |
| IDLH (Immediate danger) | Not listed. |
| Related compounds | |
| Related compounds |
Morpholine Benzomorpholine hydrochloride Tetrahydroisoquinoline Isoquinoline Quinoline |