Benzimidazole-2-thiol’s story goes back to the early research on heterocycles in the twentieth century, during a time when chemists chased better understanding of nitrogen-containing rings for dyes, drugs, and synthetic intermediates. This compound took shape alongside the rush for new therapeutic agents in the 1940s, where scaffold modifications led to breakthroughs in pharmaceuticals. Over the years, its structure became a key platform for chemists exploring antibiotics, antifungals, and anticancer candidates. Researchers found the sulfur at the second position brought a different reactivity, making benzimidazole-2-thiol a springboard for work in both academic and industrial labs. Learning the nuances of its behavior shaped not only modern synthetic chemistry, but also pharmaceutical innovation.
The laboratory bench sees benzimidazole-2-thiol as a white to pale yellow crystalline powder, with a distinct odor. Its uses stretch from basic research to the manufacturing floor—in drug discovery, dye synthesis, and as an intermediate for agricultural chemicals. Chemists value it for the strong nucleophilicity the thiol group brings, and for how the benzimidazole framework can slip into biological systems, fitting receptor shapes like a key in a lock. Product labels often highlight its purity level and moisture content, which impact reactivity in sensitive synthesis or formulation processes.
This compound stands out for a melting point close to 300°C, showing it tolerates the heat of many standard reactions. Its solubility profile tells much about its applications—it dissolves partially in water, more freely in organic solvents like ethanol or dimethyl sulfoxide. Its stable aromatic core faces little decomposition in air but remains sensitive to strong oxidizers because of the thiol. The pale yellow hue comes from subtle electronic interactions in the benzimidazole ring. It’s not volatile under standard conditions, so it stays where it’s needed, which helps in laboratory safety.
Most reputable suppliers offer benzimidazole-2-thiol at purities greater than 98%, along with detailed certificates of analysis that document heavy metal levels, water by Karl Fischer titration, and structural identity through NMR or MS. Packaging reflects its air-sensitive thiol, often double-bagged and sealed tight to block moisture intrusion. Labels always urge storage in a cool, dry place away from light, with hazard statements reminding chemists about sulfhydryl irritancy and the dust’s potential for respiratory irritation. Batch numbers and manufacturing date traceability back up recalls or regulatory inquiries.
One cornerstone synthesis routes through o-phenylenediamine and carbon disulfide in alkaline medium, yielding benzimidazole-2-thiol after cyclization and acidification. Some methods tweak the base or solvent to drive purity or improve yield. I’ve found temperature control critical—run too hot and impurity profiles climb; too cold, and conversion lags. Scale-up from flask to reactor brings challenges with mixing and exotherm management, but well-tuned process control delivers reproducible product suitable for downstream derivatization or formulation.
Benzimidazole-2-thiol’s thiol group attracts alkylation and acylation attempts, opening doors for structure-activity studies in pharmaceutical research. I’ve run reactions with alkyl halides to make thioethers, and the difference in biological properties sometimes jumps out in small screening panels. The benzimidazole ring holds up well to electrophilic substitutions—bromination or chlorination on specific positions, followed by nucleophilic substitutions that generate custom building blocks for medicinal chemistry. Oxidation of the thiol to sulfonic acid or disulfide comes up in work on antitumor and fungicidal agents. These reactions turn the core scaffold into a chemist’s playground for diversity.
Across the globe and literature, benzimidazole-2-thiol pops up under several names. Chemists may call it 2-mercaptobenzimidazole or mention commercial trade names in rubber compounding. Regulatory filings might use 2-benzimidazolethiol, and in patent dossiers, you can find abbreviations or systematic nomenclature. Getting familiar with these synonyms spares confusion in ordering or safety lookup, especially when reviewing international safety data sheets or customs documentation.
Working with benzimidazole-2-thiol involves strict attention to ventilation. The dust gets under the skin of unprotected hands, causing rashes or allergic sensitization. I always use gloves, goggles, and a dust mask, especially when weighing out charges in the synthetic lab. Disposal routes classify waste as hazardous—you can’t pour thiol-bearing slurries down regular drains without running afoul of environmental rules. Some countries limit allowable exposure in workplaces due to chronic toxicity linked in animal tests. The community benefits from rigorous risk assessment, eye-rinsing stations, and strong hazard communication for everyone on the handling crew.
Beyond the bench, benzimidazole-2-thiol made its mark in the rubber industry. As a vulcanization accelerator, it cut curing times, sharpened product quality and gave manufacturers finer control over physical properties. Agrochemical chemists look to its core for new fungicide scaffolds, since the sulfur hits fungal targets in ways simple benzimidazoles miss. Medicine saw it rise as both an intermediate and a structural analog for antiulcer, antiviral, or antihypertensive agents. Analytical chemistry uses the sulfur atom for complexation studies and detection protocols in trace metal analysis. The flexibility of benzimidazole-2-thiol brings innovation wherever sulfur and nitrogen together start promising chemical stories.
Ongoing research follows several streams. In pharma, substitution on the ring or at the thiol tail produces libraries for high-throughput screening campaigns, hunting for new activities against infectious diseases or cancer. Materials science takes advantage of the sulfur for coordination polymers, and for sensors seeking selective detection of metals or reactive oxygen species. Polymer chemists graft variants onto backbones to create responsive materials or drug-delivery vehicles. Each tweak gets followed by computational studies and bench-scale screening, building up a map of physicochemical properties with real-world consequences.
Benzimidazole-2-thiol doesn’t escape toxicity scrutiny, as sulfur-containing aromatics tend to raise regulatory eyebrows. Oral, dermal, and inhalation studies in rodents show liver and kidney impacts at high doses. Some metabolites trigger immune responses, which steers handling and packaging best practices. In vitro studies suggest the compound’s reactivity can break DNA strands, earning it mutagenicity tests and monitoring in pharmaceutical process waste. These data keep showing up in REACH and OSHA filings, pressing the industry to design safer analogs or deploy engineering controls to bring staff exposure as close to zero as practical.
Benzimidazole-2-thiol faces a crossroads. Synthetic chemistry keeps finding new ways to spin its structure into medicines, materials, or agricultural agents. Green chemistry principles push the hunt for cleaner syntheses, higher atom economy, and recyclable catalysts. Toxicity triggers requirements for more selective action in the field, and regulations set tighter allowable environmental residues. Automation and in silico prediction open up chances to screen virtual analogs before ever heading to the bench. In my experience, industries that adapt—focusing on safer, smarter, more sustainable chemistry—will unleash benzimidazole-2-thiol’s hidden potential for another generation of breakthroughs.
Benzimidazole-2-thiol takes on a pretty big role in medicine. Doctors prescribe a good number of drugs built off its core structure. Research backs this up—a 2022 review in European Journal of Medicinal Chemistry called benzimidazoles “versatile tools” in the fight against bacterial and fungal infections. I spent a lot of time shadowing a hospital pharmacist, and saw plenty of antifungals and antiparasitics derived from this group. Take albendazole, for example. It clears out intestinal worms that otherwise sap energy and concentration, especially in kids. Without these meds, basic health care would take a major hit in many parts of the world.
The chemical world uses benzimidazole-2-thiol as a starting point for building bigger, more complex molecules. Anyone who’s set foot in a synthetic chemistry lab knows you won’t get decent outputs without stable scaffolds and the right starting blocks. This compound’s sulfur atom offers a key site for reactions. Chemists often rely on it to craft dyes, photosensitive materials, or agents that prevent metal corrosion. In my undergraduate lab days, I worked on a project targeting new organic dyes, and benzimidazole derivatives came up often because of their strong electron-donating features and stability.
Farmers turn to benzimidazole-based fungicides to keep crops healthy, especially in regions where fungus and blight regularly wipe out harvests. This compound provides a strong backbone that helps synthesize fungicides with notable staying power. Overreliance can lead to resistance problems, though. Studies from the Journal of Agricultural and Food Chemistry point to “emerging breakdown of benzimidazole treatments” in some crops. As fields face climate shifts and stronger pathogens, research teams worldwide keep tweaking the core structure in search of new solutions. On my uncle’s soybean farm, I remember the yearly scramble to pick fungicides that still worked—sometimes the older benzimidazoles stopped giving results, and it took a switch to keep the crop safe.
Factories often add benzimidazole-2-thiol to lubricants and rubber compounds. This chemical shields equipment from rust and wear, helping everything last longer under tough factory conditions. The tire industry, for example, counts on antioxidants based on this series to withstand heat and sunlight. According to a 2023 global market report, the demand for these antioxidants has stayed steady even as other synthetic additives cycle in and out. Plant managers I got to know in the Midwest said you can run a batch of production with the usual wear, but without proper protection, downtime from corroded parts eats up hours and slices into profits.
The uses of benzimidazole-2-thiol reach well past textbooks and theory. From fighting disease to saving crops and keeping machines running smoothly, it delivers plenty of value. That said, there’s work left to do. Resistance in crops and pathogens grows, and industry needs greener, safer manufacturing processes. Teams in both academia and business push for less toxic derivatives. Cleaner synthesis routes, like enzyme-based catalysis, offer hope for scaling these chemicals with less waste. Science doesn’t stop with the basics—every new generation of benzimidazole-2-thiol products brings an extra layer of possibility, both in labs and the world beyond.
Benzimidazole-2-thiol looks simple but packs a punch. Chemically, it combines a fused bicyclic ring—where a benzene ring joins with an imidazole ring—and adds a thiol (–SH) group at the second position of the imidazole. The molecular formula, C7H6N2S, tells a story of structure and function. Two nitrogen atoms in the imidazole ring help the molecule interact with metals and biological targets, offering opportunities and challenges all at once. The presence of the sulfur atom is particularly important; it brings a reactive handle. Thiols wind up celebrated for their strong nucleophilic behavior, which often increases biological activity.
Researchers and industry professionals encounter benzimidazole-2-thiol across several fields. Medicinal chemists pay attention to this structure for a good reason. Doctors prescribed related molecules for their antiviral, antibacterial, and antifungal abilities. Farmers get support from it, too, through the design of new agrochemicals that limit crop loss without fouling up groundwater supplies. I’ve seen benzimidazole derivatives show up in lab work focused on drug resistance. These compounds help disrupt enzyme activity by binding tightly to metal centers, which slows down the bacteria’s ability to survive antibiotics.
Benzimidazole-2-thiol is not entirely risk-free. Problems arise when disposal protocols fall short. The thiol group does more than inspire chemical transformations; it can also make the molecule toxic in aquatic environments. Municipal water supplies can’t always filter out traces of these chemical building blocks. Exposure to animals or humans at high concentrations raises health concerns, with skin sensitization and organ toxicity at the forefront.
This chemical’s persistence in the environment gives me pause. One study published in the journal Environmental Science and Pollution Research highlighted how derivatives reach water systems and then accumulate in sediment. That is not an abstract problem—too many riverbeds now serve as chemical repositories.
Stronger regulations help, but practical solutions depend on better awareness and tighter lab procedures. During my time in graduate school, labs recognized that accidents and spills happened far too often when teams weren’t regular about safety checks and proper labeling. Enforcement of chemical waste collection, coupled with green chemistry approaches, makes a real impact.
Green chemistry aims for reactions that reduce dangerous byproducts. By tweaking the synthesis of benzimidazole-2-thiol—for example, using water-based solvents and choosing less hazardous sulfur sources—researchers make strides in protecting workers and the planet alike. It’s also possible to design filter technology that captures thiol-containing molecules before they run off into the environment. Some academic labs in Europe started collaborating with municipal wastewater plants, developing pilot projects that show measurable drops in contamination after just a few months.
Benzimidazole-2-thiol will stay popular in research labs, manufacturing plants, and pharmaceutical pipelines. Then the question becomes: how do we strike a balance between chemical usefulness and environmental safety? Prioritizing proper handling, investing in green innovation, and keeping a close eye on environmental impacts offers the clearest path forward. These choices do not make headlines, but they decide the kind of footprint we leave behind.
Benzimidazole-2-thiol pops up in labs, industrial sites, and academic settings. Most people outside of research and manufacturing haven’t heard of it, but those handling chemicals see it as a tool, not just a formula on a label. Looking beyond the jargon, it’s a compound worth treating with caution. Many experience a strong-smelling powder, sort of like rotten eggs due to the sulfur. That alone hints at how powerful and reactive it can be.
Many chemists treat benzimidazole-2-thiol with gloves and goggles, not because they're overcautious, but because there’s real risk with skin and eye exposure. Breathing in dust often brings headaches or throat irritation after just a few minutes in a poorly ventilated room. Animal tests suggest more serious risks, such as liver and kidney trouble when exposure goes on unchecked. There’s also evidence of genetic mutation in lab setups, pointing to possible long-term effects that go beyond a simple rash or cough.
Spill a bit of benzimidazole-2-thiol in a lab sink, and you’ll never forget the anxiety. Wastewater systems aren’t built to neutralize these molecules, so a little can linger in the environment. A study in the journal "Ecotoxicology" found that this compound hangs around in soil and doesn’t break down quickly. That makes it a real worry if it finds its way into lakes, streams, or groundwater used for farming. Crops can take up residues, possibly affecting both people and animals down the food chain. Unlike materials you wash away and forget, this one leaves its mark.
Benzimidazole-2-thiol isn’t radioactive or immediately fatal in tiny amounts, but the way it harms cells means small exposures add up. International agencies such as the European Chemicals Agency classify it as “harmful if swallowed,” causing eye and skin irritation, and possibly harming fertility. The NIOSH guide puts it under chemicals you don’t want to breathe often. People with asthma or skin problems end up feeling worse after exposure, especially without proper gear.
No badge or title changes the danger—technicians, cleaning staff, and researchers all face the same risks. Training and safety data sheets matter more than most offices admit. During my time handling chemicals, stories kept repeating: someone tried to shortcut the mask, or a spill went ignored, leading to burns that took weeks to heal. These weren’t clumsy people—they just worked under pressure or underestimated the risk.
Real improvements come through proper storage, working vents, and labs that budget for both fume hoods and good gloves. Everyone benefits when cleanup protocols don’t live just on paper but in daily habits. Using a substitute sounds easy until you dig into cost and performance, but safer alternatives exist for some applications. Industry won’t turn away from useful chemicals overnight, so education works better than bans.
The conversation needs to shift from individual blame to collective responsibility. Keeping benzimidazole-2-thiol in check doesn’t just protect workers but everyone in the wider environment. A quick review of training, regular equipment checks, and sharing updates about safer practices build a culture where science thrives without hidden hazards.
Over years of handling specialty chemicals, I’ve noticed that the finest details—like storage—make all the difference between smooth lab work and risky surprises. Benzimidazole-2-thiol, sometimes found in research labs, pigment synthesis, and pharmaceutical development, brings along specific handling cues that should not be ignored.
Moisture has a knack for inviting trouble with powdery organics. One accidental spill or a leaky cap invites water in, and that means degradation, clumping, or even unwanted reactions. A low-humidity environment helps a lot. For benzimidazole-2-thiol, I recommend keeping it in a tightly sealed container, stored in a cool place—well away from anything damp, and far from direct sunlight. Direct light can sometimes nudge certain sulfur compounds toward breakdown, and it can get warm near the window, even in storage. I once left a similar compound sitting on a benchtop exposed to sunlight for a week and found an unmistakable yellow discoloration. That confirmed for me: Light exposure really changes things.
It’s easy to cut corners with labeling when you’re busy. Still, labeling and dating every bottle or jar makes for fewer headaches later. Labels peel or fade, so I use lab tape and a permanent marker. If an old jar gets left for months, you will want to know what’s inside, how old it is, and whether it belongs back on a shelf or in hazardous waste.
Double containment also pays off if benzimidazole-2-thiol sits on a shared shelf. A screw-cap bottle placed inside a zip-bag has stopped minor spills from turning into major cleanups more than once in my work.
Benzimidazole-2-thiol’s sulfur content puts it on my list of “keep away from oxidizers.” Mixing sulfur organics with strong oxidizers (like nitric or perchloric acid) can lead to dangerous situations. Make a habit of setting aside a separate storage area in your chemical cabinet—a different shelf or a closed compartment does the trick.
If you ever open your storage space and pick up an odd, pungent smell, that’s a sign you should check your chemical bottles for leaks or degrading material. Ventilated cabinets help manage any unwanted fumes, and they protect everyone working nearby.
Wearing gloves, eye protection, and a mask gives real protection, not just peace of mind. If you live with kids or pets, make sure your work area stays locked and labeled. The same rules apply in any shared workplace: Never leave open containers unattended, and always put materials back where they belong—right after use.
If you need to toss expired or unwanted benzimidazole-2-thiol, contact your lab’s environmental health office, or a local hazardous waste facility. Never rinse it down a drain or toss it in the trash; it lasts much too long in the environment and poses risks for water and soil.
Being careful with chemical storage isn’t just some item on a checklist—it is a daily routine that saves equipment, experiments, and sometimes lives. Keeping benzimidazole-2-thiol cool, dry, sealed, and separate goes far beyond regulations. It’s a habit that protects everyone who shares your workspace and helps you keep your research or project on track.
A scientist staring at a chemical order sheet looks for one detail above all: the purity percentage. For Benzimidazole-2-Thiol, this one figure often tips the scales between purchase and rejection. Most suppliers offer it at 98% or above, sometimes as high as 99%, with careful notation of both assay methods and any residual content. That percentage isn’t just fine print — it carries serious weight in the lab.
People who work with organic molecules know tiny variations change whole outcomes. Impurities like water, inorganic salts, or related benzimidazole derivatives can block reactions or trigger side products. An organic chemist driving a synthesis route for a new pharmaceutical intermediate depends on a predictable compound. If the purity dips even one percent, the reaction yield may suffer, or the necessary isolation steps multiply. I learned the hard way during a grad school project. My crude NMR showed ghosts I hadn’t planned for, traced to a supposedly “pure” thiol that was just shy of the published 99%. Cleaning up downstream was a headache and a half.
Reputable suppliers attach a certificate of analysis with every bottle. Standard methods — HPLC, GC, sometimes TLC — back up the purity claim. The analysis lists not just main compound content but also moisture and any residual solvents from synthesis. Some labs rely on in-house checks, especially for scale-ups. No one likes being halfway through a multi-step project only to find out their starting material came spiked with non-obvious fillers or old solvent.
For research grade, 98% usually qualifies as “pure.” Fine chemicals intended for pharmaceutical or electronic uses often get a higher bar — 99% plus, dried and stored with desiccants, handled under inert gases. Lower-purity versions sometimes pop up, generally priced for applications where slight contamination won’t matter, like basic reagents for teaching labs or crude process trials.
Contaminants sometimes cause bigger headaches than folks expect. Metal residues, for instance, sneak in from catalysts or vessels and trip up catalysts later. Even extra water can mess with some reactions, especially those needing anhydrous setups. My own work in sulfur chemistry constantly ran up against the need to maintain “clean” batches, since trace oxidants in low-purity thiol led to discoloration or failed isolations. It’s tempting to go for a bargain, but downstream costs almost always outweigh the upfront savings.
A clear solution involves greater transparency from suppliers. Listing detailed batch analyses on product pages, updating storage and handling guidance, and flagging known contaminants all help buyers make informed decisions. Labs, especially those on tight budgets, sometimes resort to re-purifying material themselves. Crystallization or charcoal treatments add time and labor. Some suppliers now offer custom purification, and that trend could become the norm as demand grows for higher quality in both industry and academia.
To anyone considering Benzimidazole-2-Thiol for their next synthesis: read the certificate, check the assay method, know your application, and don’t cut corners on purity for price. Saving a few dollars doesn’t mean much if the whole experiment gets derailed by a contaminant hiding in the batch.
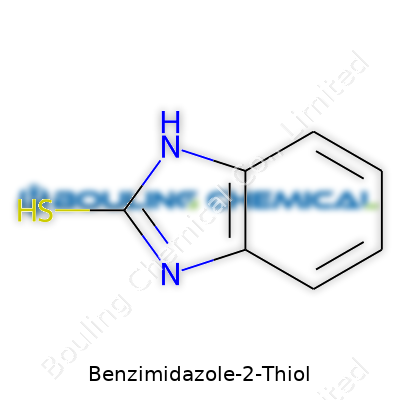
| Names | |
| Preferred IUPAC name | 1,3-dihydro-2H-benzimidazole-2-thione |
| Other names |
2-Mercaptobenzimidazole 2-Benzimidazolethiol Mercaptobenzimidazole 1H-Benzimidazole-2-thiol |
| Pronunciation | /ˌbɛn.zɪˈmɪd.ə.zəʊl tuː ˈθaɪ.ɒl/ |
| Identifiers | |
| CAS Number | 238-76-0 |
| Beilstein Reference | 120908 |
| ChEBI | CHEBI:17408 |
| ChEMBL | CHEMBL424045 |
| ChemSpider | 73024 |
| DrugBank | DB12983 |
| ECHA InfoCard | 15a2de9f-11da-47ee-ba09-e5ef437d7f17 |
| EC Number | 283-464-9 |
| Gmelin Reference | 72622 |
| KEGG | C06565 |
| MeSH | D007398 |
| PubChem CID | 9274 |
| RTECS number | DM1225000 |
| UNII | 2XY69VXR5R |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C7H6N2S |
| Molar mass | 150.20 g/mol |
| Appearance | Yellow crystalline powder |
| Odor | Odorless |
| Density | 1.293 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.58 |
| Vapor pressure | 4.2 x 10^-6 mmHg (25°C) |
| Acidity (pKa) | 5.55 |
| Basicity (pKb) | 11.4 |
| Magnetic susceptibility (χ) | -69.0×10^-6 cm³/mol |
| Refractive index (nD) | 1.730 |
| Dipole moment | 3.62 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 126.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -91.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -5617 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin and eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| Precautionary statements | P261, P264, P270, P271, P273, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | Flash point: 158°C |
| Lethal dose or concentration | Oral Rat LD50: 400 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 640 mg/kg |
| NIOSH | LTQ7540000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10~50 mg |
| Related compounds | |
| Related compounds |
Benzimidazole 2-Mercaptobenzimidazole Benzimidazole-2-thione 1H-Benzimidazole-2-thiol 2-(Methylthio)benzimidazole |