Chemistry has a way of surprising even those who think they’ve seen it all. Stretching back to the late 19th century, Gerhardt and his colleagues poked around sulfur- and nitrogen-containing rings, which paved the road to thiazole chemistry. 2-Aminothiazole—shorthand: 2-AT or thiazol-2-ylamine—emerged as researchers in the early 1900s started looking for compounds fitting both the medicine cabinet and the industrial toolkit. This molecule became a strong asset for folks chasing new drugs, pesticides, and dyes, especially after German chemists realized its knack for binding to proteins and changing biological activity. These early threads spun into a pattern: 2-Aminothiazole became a mainstay for labs the world over looking to invent what hadn’t come before.
2-Aminothiazole looks like a pale yellow powder. Run it through standard tests, and it stands out for solubility in hot water and polar solvents such as methanol, ethanol, or acetonitrile. Its melting point reads around 127 to 130°C. Smell it, you’ll catch a hint of sulfur—nothing floral here. Chemical suppliers stock it up in several forms, including technical grade and high purity stuff for pharmaceutical routes. With its five-membered thiazole ring and amine group hanging on the second carbon, chemists see it as a sturdy spot to tack on new pieces, making it a staple for synthesis routes aiming for more ambitious chemical architectures.
2-Aminothiazole packs a punch for its size. With a molecular formula of C3H4N2S, its molar mass sits at 100.14 g/mol. The ring structure, bearing sulfur at the one position and nitrogen at the three, creates both electron-rich and poor areas. This split personality lets it jump into reactions both as a nucleophile and as an electrophile. As a solid, it resists light and stays stable under normal lab conditions. Toss it in water above room temperature and it’ll dissolve; try nonpolar solvents, and it hardly budges. In the presence of acids, the amine group can grab up a proton, shifting reactivity. High temperatures or strong bases will start to poke holes in the ring and break it down. Its chemical backbone makes it resilient in most storage and handling conditions in the lab—barring extremes.
A bottle labeled 2-Aminothiazole usually lists its CAS number—96-50-4—along with assay (typical for research grade is above 98%), appearance, water content, and melting range. Safety labeling follows GHS guidelines: irritant pictograms, statements for skin and eye contact, and warnings about dust inhalation. Shipping containers sport tamper-proof seals and batch IDs. Shelf-life, under standard conditions, runs a good two years, though specs want cool, dry storage, a warning echoed in both safety and quality documentation. The label also mentions sample size, storage recommendations, and UN number if transport is international. Each bottle comes with a certificate of analysis, providing analytical data on purity and byproducts checked using NMR, FTIR, and HPLC methods.
Methods for producing 2-Aminothiazole often begin with boiling up alpha-halocarbonyl compounds and thiourea in alcohol or water. This straightforward condensation doesn’t need exotic reagents or ultra-high pressures, which makes large-scale synthesis affordable. The mechanism chugs along as the thiourea's sulfur attacks the electrophilic carbon, cyclizing into the thiazole ring, then releasing the leftover salt. For example, bromoacetone reacts with thiourea in aqueous ethanol, after which filtration and recrystallization finish the job. One run in undergraduate organic lab feels like a rite of passage, and scaling to kilo batches just means slightly bigger flasks and tougher extraction steps to handle byproducts. This approach pops up in both commercial and academic protocols, nesting into multi-step syntheses of more complicated molecules.
2-Aminothiazole acts as a playground for synthetic chemists. The amine group tacked to the thiazole ring’s C2 carbon jumps into acylation, alkylation, or diazotization reactions—opening up whole families of derivative compounds. Chemists often tack on bulkier groups using acyl or sulfonyl chlorides, crafting intermediates for antibiotics, dyes, or even novel materials. Bromination or halogenation introduces reactive handles needed for coupling reactions. Cross-coupling with aryl halides and Suzuki reactions build out bigger, more complex molecules that matter for medicinal chemistry. Nucleophilic attack on electrophilic partners or cyclization with carbonyl compounds opens the door for fused heterocyclic molecules. In hands-on terms, it’s like starting a soup with a rich base—the ways you can spice it up are endless.
Call it 2-aminothiazole, thiazol-2-ylamine, or 2-thiazolylamine—chemical suppliers list all three, along with the registry number 96-50-4. You might spot it in academic or patent literature under these labels, or even shorthand like 2-AT. Pharmaceuticals and agrochemical companies sometimes hide the name under proprietary codes or as an intermediate in multi-step syntheses, but the structure remains the same. This patchwork of names can confuse newcomers, but experienced chemists quickly learn to cross-check with molecular formula or CAS. No matter what the bottle reads, it’s the five-membered ring, not the label, that does the work.
Handle with respect—2-Aminothiazole carries moderate toxicity. Skin or eye contact stings and causes irritation; inhaled dust can set off coughing or sneezing. Prolonged exposure at high concentrations may affect the liver or kidneys, which prompted agencies to classify exposure hazards for workers in labs and plants. Standard PPE—gloves, goggles, good ventilation—forms the baseline defense. MSDS paperwork flags risk phrases for acute toxicity and environmental hazards if spilled in bulk. Waste disposal calls for incineration or collection by chemical waste contractors. Labs running syntheses use fume hoods and enforce restricted access policies, not from paranoia, but from hard-won experience about keeping trouble at bay. Training on spill response and first aid stays current, forming an unflashy but necessary safety net for anyone wrestling with bottles full of the compound.
2-Aminothiazole gets its hands dirty in all kinds of industries. Medicinal chemistry leans hard on its potential as an antifungal and antibacterial agent—the backbone structure slips into all sorts of drugs, including some used for treating tuberculosis. Agrochemical companies cook it up as an ingredient in pesticides and fungicides to protect crops without going nuclear on wildlife. Dye manufacturers use it as a building block for colorants used on fabrics, sometimes boosting colorfastness and stability. Polymer chemistry borrows it as a monomer or an additive, chasing new mechanical or electronic properties. In analytical chemistry, it pops up as a chelating agent for detecting trace metals during assays. Every time I’ve crossed into a new field, this reliable ring shows up, pulling its own weight while blending into broader chemical families.
Academic and industry labs seem locked in an arms race to find new uses for 2-Aminothiazole derivatives. Recent push in pharmaceutical R&D focuses on tweaking the core structure to improve selectivity and lower toxicity. Medicinal chemists hunt for new antimicrobials, antivirals, and antitumor agents, spinning out patents as fast as they can test compounds. Material scientists reach for 2-AT as a foundation for organic semiconductors, adhesives, or heat-resistant plastics. Machine learning and AI pop up, crunching databases to predict which new derivatives might hit targets with precision. Green chemistry researchers rework classic synthesis routes to cut out toxic solvents and lower environmental footprint—the economic and social push for “greener” approaches isn’t going away soon. In my experience working on heterocyclic scaffolds, the number of times 2-AT shows up in new grants and patent races borders on uncanny.
Toxicologists take a hard look at 2-Aminothiazole, not just for workers handling the pure stuff but also for consumers exposed to trace levels in food or water from pesticides. Acute exposure can trigger headaches, nausea, or liver enzyme changes in test animals. Chronic studies on rats support a “handle with caution” approach—no panic, but no complacency. Regulatory agencies keep updated limits on environmental discharge and residue in food crops. New toxicology research tracks metabolites and breakdown products, since some might turn out nastier than the parent molecule. Developers working on new drugs using the thiazole skeleton add extra screening steps to weed out problematic toxicity as early in the process as possible, balancing risk and reward in the push for new solutions.
Every time chemical tools evolve, 2-Aminothiazole picks up a fresh lease on life. Artificial intelligence looks set to speed up the hunt for new derivatives by predicting biological activity from millions of possible tweaks to the core. Green chemistry goals push for safer, less wasteful synthesis, cutting down on reagents and solvent use. Drug discovery faces fresh resistance threats from bacteria ignoring conventional treatments, so new antibiotics with a 2-AT backbone will keep getting a hard look. Material researchers see potential for the thiazole ring in organic LEDs, sensors, or even flexible electronics. Across all this, demand for careful safety standards and more transparent toxicity data grows—with stricter rules and digital recordkeeping tightening up practices from lab bench to global shipment. It’s the sort of workhorse chemical that doesn’t fade with trends, but instead evolves alongside the people who keep reaching for it, decade after decade.
Chemical names tend to shut down half the brains in a room, but 2-Aminothiazole isn’t just another line in a catalog. In real-world labs, chemists pull it off the shelf more than most would guess. For drug development, this compound offers a door into building new and better medicines. It's got a knack for locking into biological targets. Pfizer, Bayer, and many other pharmaceutical giants give thiazole rings attention for one reason: they help fight infection and inflammation and attack cancer cells.
Those who work in synthesis know the game—finding a scaffold with proven bioactivity makes a tough job easier. 2-Aminothiazole fits the bill. Scientists have leaned on it while cooking up antibiotics to tackle those tough bacteria strains that laugh at old drugs. It also gets mixed in with other pieces to create antifungal agents and antivirals. From my time in research, every new graduate knows these types of molecules often end up in patent filings, just because they work so well as frameworks for further development.
Doctors owe a few victories in the infectious disease space to molecules born from 2-Aminothiazole. It's no accident: this chemical sits at the starting point for cephalosporins—one of the more trusted antibiotics doctors still use when other options fail. It sneaks into anti-tuberculosis drugs, too, showing some promise where standard regimens hit a wall.
Stepping outside antibiotics, cancer researchers have found that swapping certain atoms on this ring can slow tumor growth. You see real hope in chemical review articles showing variations on this backbone that hinder enzyme pathways vital to cancer cells. Thiazole-linked small molecules often appear in the running list of experimental drugs trying to treat Alzheimer’s or even manage inflammation.
It’s easy to dismiss the significance of a single molecule with a long name, but the health system relies on relentless chemical tinkering. 2-Aminothiazole is small, simple, and stable. That mix means chemists can alter parts of the ring, testing changes with speed. In today’s pharmaceutical game, that’s the difference between catching up and missing out.
From the ground up, success depends on foundational ingredients that let researchers chase resistant bugs, persistent tumors, or undiagnosed nagging infections. I remember staring at pages and pages of failed molecules; a good scaffold, like 2-Aminothiazole, is worth more than a ten-page literature search.
No one should gloss over the tough parts. Introducing any new chemical into the body sets off questions—how toxic, how fast is it cleared, what happens in the liver? Some 2-Aminothiazole derivatives have stumbled, showing side effects or failing to outperform current medicines. Each letdown reminds us: there’s no shortcut. Safety profiles do not get a free pass, even on promising backbones.
What stands out is the need for smarter use rather than more of the same. Pushing the same building block everywhere risks resistance, especially with antibiotics. We have to keep mixing up our approaches; pairing thiazole-based molecules with new delivery systems or targeted therapy can stretch their value without putting all our eggs in one basket.
I still remember staring at a molecule sketch as a student, wishing someone would just tell me what made a compound tick. Take 2-Aminothiazole. At first glance, its structure can look like just another odd ring on the board, but each atom earns its place for a reason.
In 2-Aminothiazole, you find a five-membered ring. Half of those ring members are not even carbon. You’ve got nitrogen at the first position (N1), carbon next to it (C2), then sulfur (S3), and two more carbons (C4 and C5). The amino group sticks right onto that C2. Picture it as a house with a sulfur and nitrogen foundation, and the amino group as a quirky second-story addition. Scientists write its molecular formula as C3H4N2S. That’s three carbons, four hydrogens, two nitrogens, and one sulfur—just enough to keep things interesting but not confusing.
I’ve found that details matter in the lab and in real life. Getting the formula down—C3H4N2S—not only keeps you grounded in what’s real, but stops nasty surprises. Misjudge that blend, and you risk failed syntheses or, worse, safety slip-ups. Being precise means you hit the right dose, the right reaction path, and you cut waste.
Chemists look at its ring for good reason. Compounds built like this don’t just sit on a shelf. Thiazole rings pop up in antifungal drugs, antibiotics, dyes, and even plant protection sprays. The amino group at the 2-position is no garnish; it makes an already lively platform even more useful by opening connections for more reactions.
If you wander through a medicinal chemistry lab, there’s a decent chance you’ll spot a reaction trail with 2-Aminothiazole. There is something about small, versatile molecules that lets researchers dream big for treatments and solutions. It’s not just about creating new materials—it’s about practical results. This tiny molecule’s frame helps researchers build medicines for everything from epilepsy to fungal infections. Back in college, hitting that sweet spot between making a molecule and finding an actual use for it felt elusive. Thiazoles bring craft to that process.
The same tight-knit structure that makes 2-Aminothiazole useful also brings a bit of a challenge. Production at scale calls for safe processes because sulfur-containing rings can spring some wicked surprises. I’ve seen labs take extra steps with ventilation and containment—not optional if you want to protect health. Streamlining synthesis to cut toxic byproducts can save time and money, and protects the environment.
Sharing best practices, both for making and disposing of these compounds, lies at the core of moving from theory to real change. Learning from mistakes and smart tweaks in the process—switching to greener solvents, rethinking routes for lower waste—brings a better future all around. It starts with recognizing the basic outline of 2-Aminothiazole and then using it with respect for its power and its risks.
2-Aminothiazole often pops up in research labs, especially those exploring new drugs. It looks like a simple beige powder, but the way it’s handled behind the scenes tells a fuller story. I’ve worked with this compound for years. I’ve watched some labs throw caution out the window, treating it like table salt. A few ruined experiments and more than one irritated nose later, you learn: Even the most unassuming powder demands respect.
At first, I didn’t think much about the air in the chemical store room. It always smelled odd already, so what difference would a few grams of 2-aminothiazole make? The answer hit one day, after a warm spell rolled through and humidity crept in. The compound started clumping in its bottle. Turns out, 2-aminothiazole draws water out of the air if you give it the chance. Moisture can nudge it toward breaking down, and you end up with something less pure, less reliable.
So, a solid approach starts with tight caps and real chemical bottles. Some folks use improvised containers — hand-labeled jars, random lids that never quite fit. Don’t. The right screw-cap bottle with a seal stands between you and spoiled material. Toss in a packet of desiccant, too, just for good measure; silica gel keeps things dry, and it’s cheap insurance.
I once worked in a lab where everything went straight into the fridge — solvents, solids, snacks. People thought the colder, the better. But not every chemical likes it cold. With 2-aminothiazole, room temperature in a dark, dry spot usually does the trick. Extreme cold can pull moisture when things warm up, making storage more trouble than it’s worth. Simple shelves, out of sunlight, often provide the stability this compound craves. A chemical storage cabinet marked for organics solves the problem.
Plenty of lab stories start with someone grabbing the wrong jar. I made that mistake once — spent an afternoon cleaning tiny yellow crystals out of a reaction flask. Clear labels can save your research and your safety record. Use clear names, prep dates, expiration guesses. Quick reference means nobody’s guessing what’s inside, and nobody’s risking a splash or an inhaled puff of dust.
Over time, I’ve seen how proper air circulation makes a difference. A stuffy cabinet compounds odors, especially if leaks or spills happen. Even a tight bottle isn’t perfect. A spot with a gentle air draw keeps potential fumes at bay, especially if 2-aminothiazole shares space with other strong-smelling chemicals. If you can't set up dedicated venting, at least keep storage away from heat and busy foot traffic. Smashed bottles or knocked-over openers often happen near crowded shelves.
No one plans to spill a gram of this compound, but every chemist I know has done it. Soak up powders with damp paper — don’t sweep up dry, as dust gets everywhere. Old, degraded 2-aminothiazole belongs in the lab’s hazardous waste bin, not down the drain or mixed with trash. Keeping waste sorted means less headache when disposal day comes around, and less risk for everyone in the building.
Safe storage isn’t flashy, but it saves money, time, and sometimes health. A little planning, sensible labeling, airtight containers, and a respect for small details — that’s what keeps 2-aminothiazole doing what it’s supposed to do.
If you spend time in any chemical lab, you start to recognize not every flask or bottle poses the same threat. 2-Aminothiazole usually shows up as a pale yellow powder. It doesn’t look that intimidating, but the risk isn’t in how it sits quietly on the shelf. Breathing in the dust or dust getting on skin can cause irritation pretty fast. Eyes sting, skin itches, and your lungs can feel scratchy if you get careless. Working with it, you realize this is not the sort of powder you want blowing around like flour in your kitchen.
This chemical hates light and moisture. I've seen bottles go clumpy or change color after a couple of weeks out of the cabinet. To keep things from going south, storing 2-Aminothiazole in a sealed, dark glass jar with a tight-fitting lid works best. I always check the desiccant packs, too. People sometimes forget them, but a dry environment keeps the powder from turning useless or, worse, risky to handle.
Gloves are a must. Nitrile gloves keep my hands safe through long days, even if I don’t plan on touching anything directly. Safety goggles have saved me from splashes more times than I want to admit. Dust masks or, better, a fitted respirator, filter out the fine particles during weighing or transfer. This stuff floats up easily once you open the container, so leaning in close without protection isn’t smart. A long-sleeve lab coat and closed shoes finish the basics since spilled powder always finds unprotected skin.
Spills bring out the real trouble. Sprinkling water or, worse, solvents on the powder spreads it around and sometimes makes cleanup harder. Gentle sweeping with damp paper towels, then sealing those in a bag right away, works better. I always toss the towels in with a bit of compatible waste solvent just to keep anything from becoming airborne during disposal. Never use a standard vacuum—you don’t want dust circulating in the air.
A fume hood makes the biggest difference in air quality. I learned early on that using an open bench leads to subtle headaches and scratchy throats by the end of the day. The hood’s steady airflow whisks away the powder before it drifts. Proper habits, like taping down weighing boats and closing the bottle after every use, control 90% of the mess before it starts.
Quick rinses under running water for eyes or skin pays off if there’s a splash. I’ve kept an eyewash station nearby since my first near-miss. Washing for at least 15 minutes sounds like overkill, but it makes a difference if you want to avoid redness or lingering irritation. For inhalation, stepping outside into fresh air while calling for medical backup gets help started early if breathing trouble kicks in.
A clear, bold label on the bottle means nobody mistakes it for something harmless. Everyone working nearby should know the hazards and emergency plan before getting started. Used gloves, weigh boats, and cleaning materials go straight into hazardous waste. My lab switches out collection bins regularly, as waiting too long just invites someone to reach in carelessly.
Staff briefings set the tone for a safe lab environment, but real learning hits during everyday work. Regular checks on storage conditions keep issues from creeping up. Double-checking labels, not rushing through transfers, and wearing the right gear lowers the chance of trouble. In my experience, small steps like these make handling 2-Aminothiazole safer for everyone, whether you’re new to the lab or a seasoned tech.
2-Aminothiazole sits on the shelf in a lot of labs. Its reputation comes from its work in drug discovery, dyes, and other specialty chemicals. Even folks outside of chemistry have probably brushed up against something downstream of this compound. If you find yourself zeroing in on this molecule, odds are you’ll notice sellers offering more than one “grade” or “purity.”
Consider shopping for flour at the grocery store. Some people reach for all-purpose, others grab super-fine cake flour. Chemists face a similar aisle, only the labels say things like “technical grade,” “laboratory grade,” or “pharmaceutical grade.” These tags signal different levels of unwanted stuff in the bottle. Technical grade products usually have a few more stragglers—impurities that don’t matter for industrial jobs like pigments or bulk intermediates. Dig into a pharmaceutical-grade supply, and the purity jumps significantly, often topping 98-99%. At that level, suppliers shake out most impurities that might otherwise mess with a biological test or end up in a drug.
As someone who learned the hard way, those small impurities pack more punch than you expect. I once ordered a cheap bottle for an undergraduate experiment on a shoestring budget. The price tag looked great, but in the end, we couldn’t trust a single result. Trace contaminants completely scrambled our numbers. We spent more time troubleshooting than doing science. Lessons get expensive, even in the microgram range.
Lab workers spend most of their time chasing consistency. In research, people pay extra for the higher grades to remove wild cards from the reaction pot. Medicinal chemists—especially those feeding results into animal tests or clinical work—treat that extra 0.5% as the difference between a clean outcome and a failure report. Trace metals, leftover reagents, or water all create headaches when it comes to sensitive analyses or regulatory hurdles.
Scale changes things too. At industrial scales, companies might sacrifice purity for volume savings when it won’t cause harm or violate regulations. The same plant may keep two bins: one for small specialty batches where every molecule counts, and another for the bulk jobs where speed and cost rule the day. Neither approach is “right” in every case; context makes the call.
Government policies inevitably step in, especially at the pharmaceutical and food interface. Regulatory bodies like the FDA want clear documentation of what’s inside every bottle moving into drug production. Failing to meet these standards can stall an entire project—take the wrong batch, and you’ll spend weeks answering questions from a review board.
Transparency helps everyone. Suppliers should spell out what grades they offer, backed up by detailed certificates of analysis. Researchers and buyers can ask for data on impurities that actually matter for their work. I’ve seen groups band together to buy higher-grade chemicals in bulk and share the costs—cuts down on wasted funds and blunders. There’s also value in setting up a supply chain that tracks quality from start to finish, so each step is built on reliable data instead of guesswork.
Making the right pick comes down to your end use. If you’re mixing up a dye for textiles, you probably care more about color yield than about a stray contaminant. If you’re pushing the frontiers of drug design, you learn to sweat the small stuff, sometimes down to a tenth of a percent.
In the world of chemicals, details matter. Pretending otherwise usually means learning the hard way.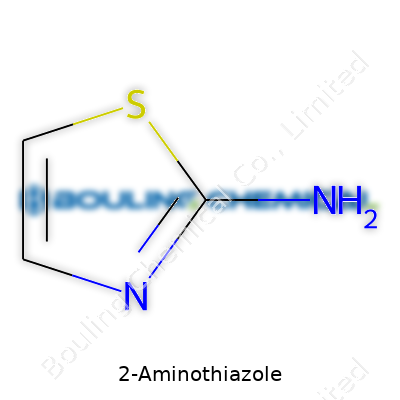
| Names | |
| Preferred IUPAC name | 1,3-Thiazol-2-amine |
| Other names |
2-Amino-1,3-thiazole 2-Thiazolamine Thiazol-2-ylamine Aminothiazole |
| Pronunciation | /tuː əˌmiːnoʊ θaɪˈæzoʊl/ |
| Identifiers | |
| CAS Number | 96-50-4 |
| Beilstein Reference | 136697 |
| ChEBI | CHEBI:140427 |
| ChEMBL | CHEMBL402 |
| ChemSpider | 2506 |
| DrugBank | DB04310 |
| ECHA InfoCard | 100.007.796 |
| EC Number | 2.5.2.7 |
| Gmelin Reference | 110093 |
| KEGG | C02259 |
| MeSH | D000088 |
| PubChem CID | 938 |
| RTECS number | XH2945000 |
| UNII | F2TCU4T3QE |
| UN number | 2811 |
| Properties | |
| Chemical formula | C3H4N2S |
| Molar mass | 99.13 g/mol |
| Appearance | White to light yellow powder |
| Odor | odorous |
| Density | 1.31 g/cm³ |
| Solubility in water | Soluble |
| log P | 0.33 |
| Vapor pressure | 0.025 mmHg (25°C) |
| Acidity (pKa) | 5.41 |
| Basicity (pKb) | 11.07 |
| Magnetic susceptibility (χ) | -66.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.670 |
| Viscosity | 1.604 cP (25°C) |
| Dipole moment | 2.96 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 121.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 86.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1458 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | J01DS01 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements of 2-Aminothiazole: ```string P261, P280, P305+P351+P338, P304+P340, P405, P501 ``` |
| NFPA 704 (fire diamond) | 2-3-0 Health:2 Fire:3 Reactivity:0 |
| Flash point | 86°C |
| Autoignition temperature | 300°C |
| Lethal dose or concentration | LD50 oral rat 1070 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 1600 mg/kg |
| NIOSH | MW3675000 |
| PEL (Permissible) | Not established |
| Related compounds | |
| Related compounds |
Thiazole 2-Amino-1,3-thiazole-4-carboxylic acid 4-Methylthiazole 2-Aminobenzothiazole 2-Mercaptothiazole |