People have tinkered with thiazole rings for generations, chipping away at the puzzles of chemistry to chase new medicines, dyes, and materials. The family history stretches back to the late 19th century after thiazole went from a curiosity to a workhorse scaffold in drug discovery. Interest in amino-substituted thiazole compounds woke up after World War II as folks pushed into new antibiotics and crop protectants. Chemists picked up the pace with 2-aminothiazole variants in the 1960s, spurred on by new lab tools and the hunt for drugs to tackle infections and inflammation. By the 1990s, 2-aminothiazole-4-acetic acid earned a spot as a versatile intermediate in both pharmaceutical and agrochemical circles. I've seen the shift as researchers favored this acid for its ability to anchor and direct complex syntheses. Labs around the world started keeping it as a stock chemical, spoken of as both an endpoint and a stepping stone.
2-Aminothiazole-4-acetic acid isn’t flashy, but in the right hands it unlocks whole classes of biological experiments or chemical syntheses. The compound’s backbone—a thiazole ring fused with an amino group at position two and an acetic acid chain on the fourth position—gives it broad utility. You’ll come across this substance as a crystalline powder, off-white to light yellow, sometimes stubborn about dissolving in water but more at ease in organic solvents. Suppliers pack it in airtight, opaque bottles to keep humidity and light from spoiling it, as exposure makes it degrade or clump. On paper and in practice, it stands out for offering a direct route into Bioactive amides, esters, and a raft of heterocyclic derivatives.
The molar mass clocks in at around 174.2 grams per mole, not far off from what many organic acids weigh. My experience matches the reports: heating it too much causes decomposition well before you hit any reasonable boiling point. It melts between 218°C and 224°C, and forms strong hydrogen bonds, which explains the tough crystals and limited solubility in neutral water. Its amino and carboxylic groups sit far enough apart that both can participate in distinct chemical reactions. Under a microscope, the crystalline grains look clean, though even small amounts of moisture from handling can change how they clump. Folks in analytical labs have no trouble verifying purity with NMR, MS, or HPLC, since the molecule stands out in most spectra. I’ve seen its stability under most lab conditions, except for long stretches under sunlight or in acidic environments—either one will eventually start to break apart the ring.
You’ll spot bottles labeled with the familiar CAS number: 703-15-1. Purity grades stretch upward from 97%, often with batch-to-batch certificates showing percent-by-weight for major impurities. Packaging ranges from sealed vials for research (250 mg to 10 g) up to kilogram containers if you’re working at a pilot scale. Labels on legitimate products include hazard warnings: risk of eye and skin irritation, and notices about storage temps usually kept between 2°C and 8°C for best shelf life. Material safety data sheets spell out the dangers of dust inhalation or skin contact—something that anyone working with it quickly learns to respect.
Synthesis often begins with 2-aminothiazole, a bench-stable starting material. Most protocols then set up a nucleophilic substitution using bromoacetic acid or its derivatives. Acid-catalyzed or base-promoted conditions (dependending on your comfort with cleanup) drive the acetic acid chain onto the ring at position four. An ice-cold water workup then separates product from excess reactant and side-products, with filtration and recrystallization tightening up purity. For scale-ups, chemists tweak the process, sometimes using greener solvents or optimizing stoichiometry, but the skeletal chemistry stays pretty consistent. The challenge is less about inventing new routes than about nudging up the yield while keeping byproducts low. Some firms have begun using continuous flow methods, making the process safer and faster.
This molecule likes to serve as a launching pad. Its amino group gets acylated or alkylated to give amides or tertiary amines, which unlock whole new families with activity against bacteria or cancer cells. Its carboxyl group opens doors for coupling reactions using EDC or DCC, turning it into esters, peptides, or even more complex heterocycles. Adding halogens or other substituents to the thiazole ring isn’t tough either—folks have built libraries with hundreds of variants by swapping side groups. My own colleagues rely on it for straightforward conjugation to carrier proteins or for linking dyes and probes for bioimaging work. The possibilities often come down to what the attached groups can do—antimicrobial properties, signaling roles, or even as intermediates in polymer production.
The registry databases list it under several names: 2-aminothiazole-4-acetic acid, 2-amino-1,3-thiazole-4-acetic acid, and simply ATA-4AA. Chemical supply catalogs sometimes cut it short as ATAA or even ATA-Ac. These names all point to the same compound, though the abbreviations often depend on the discipline—pharmaceutical chemistry versus agricultural science. Synonym confusion occasionally causes headaches during ordering, so careful double-checking with the CAS number keeps shipments correct and research on track.
Labs using this acid don’t treat it lightly. Gloves, goggles, and local ventilation are the minimum. Powder spills chat with lungs or mucous membranes fast, so nobody wants to handle it outside a fume hood. Cleanup involves copious water or buffer solution, since dry sweeping risks sending dust airborne. Storage means dry cabinets, away from oxidizers and out of sunlight, even for samples kept temporarily at the bench. I’ve seen more than one cautionary story from colleagues who underestimated the eye irritation or burned skin from undiluted samples. While it doesn't pack the acute dangers of some reagents, respect for its MSDS isn’t optional.
2-Aminothiazole-4-acetic acid seems to show up everywhere researchers need a thiazole core. Drug developers turn to it for antibacterial and antifungal leads, modifying both amino and acid groups to tune pharmacokinetics. Agrochemical teams use its derivatives for seed coatings or crop protection, chasing better yields with less toxic residue. It’s turned up in dye chemistry, thanks to strong bathochromic shifts and stable colorants. In the academic world, students and postdocs test its reactions with new catalysts, using it as a practical substrate to teach reaction optimization. And in the medical diagnostics sector, specialists link the acid to fluorescent tags for bioassays, making use of the reliable conjugation chemistry and the molecule’s compact, rigid shape.
Current R&D focuses on new drugs, especially as standard antibiotics lose their punch against resistant pathogens. A recent wave of work uses 2-aminothiazole-4-acetic acid to build libraries of inhibitors targeting kinases and metabolic enzymes. These new compounds take advantage of the thiazole core’s ability to mimic natural substrates, earning attention at conferences and in patent filings. Some teams are engineering the molecule as a key step in peptide-based drug candidates, counting on its acetic acid chain to boost solubility and bioavailability. I’ve seen real progress in scaling up greener syntheses, swapping out old solvents for water or ethanol and finding more efficient reaction paths. Quantum chemists and theoretical biologists test docking predictions with 2-aminothiazole-4-acetic derivatives, feeding back hits to synthetic teams in a feedback loop that shortens development time.
Testing so far puts 2-aminothiazole-4-acetic acid in the “handle with care” category but not as a major poison. Acute toxicity in lab animals comes in higher than for more notorious chemicals, though chronic effects still raise questions. Research zeroes in on metabolic breakdown products—thiazole fragments can sometimes provoke unwanted cell responses or oxidative stress. Environmental fate shows some breakdown after days in water or soil, but researchers push for more complete data about long-term safety. Most researchers I know avoid direct skin contact and follow standard practices, understanding that toxicology studies are ongoing and gaps remain. Industry groups keep funding more expansive studies to answer regulatory concerns, especially as the compound heads toward larger scale applications.
There's no sign of research interest tapering off. Demand tracks with both new synthetic routes and revived focus on heterocyclic scaffolds for next-generation drugs and agrochemicals. Ongoing advances in automated synthesis and green chemistry should nudge production toward safer and more affordable routes, making it easier for startups or university labs to work with the compound. Regulatory bodies keep sharpening their standards, so clear data on toxicity and breakdown will shape how the substance enters the market. If resources stay committed to cleaner, more secure synthetic methods, 2-aminothiazole-4-acetic acid won’t just stick around—it’ll fuel another round of innovation in both the chemical and life sciences.
This chemical’s name twists the tongue. Acetic Acid 2-Aminothiazole-4-Acetic Acid doesn’t roll off the lips like aspirin or caffeine, but plenty goes on behind the scenes. I’ve seen countless researchers, especially in pharmaceutical development, reach for compounds with long, complex names like this because of what they unlock in the world of medicine.
Folks in the pharmaceutical world rely on molecules shaped just like this for more than their names. Some call it a building block; others say it’s a starting point. Ask any medicinal chemist, and they’ll point out—these small pieces get slotted into bigger compounds, often designed to fight infections. That thiazole ring, for instance, showed promise for decades in antimicrobial projects. Acetic Acid 2-Aminothiazole-4-Acetic Acid often ends up as a key part of labs developing antibiotics or antifungal agents, not on its own, but stitched into something larger with real therapeutic value.
In my own stretch dabbling in early-stage drug research, I remember the shelves lined with dusty bottles of similar compounds. The team saw them as puzzle pieces. Synthesize a handful of new candidates by tweaking these core molecules, then run them against bacteria that keep doctors up at night. If the combination looked promising, things moved forward. In many instances, the base chemicals quietly did their job—unnoticed, but fundamental to any results.
What hits home for me is thinking about the struggles to find new medicines, especially as resistance takes away old antibiotics’ punch. There’s only so much one can do with the basics. Researchers have to try new frameworks, fresh chemical scaffolds. 2-Aminothiazole-4-acetic acid structures offer a unique backbone, letting chemists graft different groups onto the molecule. The hope is that one of these modifications might break through bacterial defenses, tackle new strains, or target a unique enzyme.
A study a few years back in the Journal of Medicinal Chemistry highlighted how aminothiazole rings increased effectiveness for some antibiotic families. There’s something about these structures, perhaps in the way they stack or bond, that lets them crash through microbial barriers where traditional penicillins floundered.
There’s not much public glamour in these intermediate compounds. You won’t see their names on drugstore shelves. They come up, though, every time someone tries to leap past current limits in drug resistance or chronic infection treatments. What’s striking here: the fight against superbugs relies less on headline molecules than it does on the unassuming chemistry taking place one shelf at a time.
If there’s a challenge, it’s that too many people outside the research world shrug at these basics. It’s not something politicians take up. Funding usually focuses on results. But without the workhorse compounds like Acetic Acid 2-Aminothiazole-4-Acetic Acid, nothing gets off the ground. Support for early-stage research—grants for core chemical development, funding for independent labs—keeps the discovery engine running. Skipping this step leaves medicine stuck with yesterday’s tools.
For the world outside the lab, the work looks boring. For anyone stuck waiting for the next round of antibiotics—or a doctor facing one infection too many—these building blocks quietly shape the future of treatment. I’ve found that patience, not glamour, makes the medical world tick over.
Acetic Acid 2-Aminothiazole-4-Acetic Acid isn’t the kind of name that rolls off the tongue at a dinner table, but it shows up in research labs often enough. In straightforward terms, think of this compound as a fusion between two workhorse structures in organic chemistry: thiazole and acetic acid.
Let’s break it down. Thiazole brings a five-membered ring, sort of like a compact steering wheel made up of three carbon atoms, a nitrogen, and a sulfur. Now, stick an amino group at position two and an acetic acid group at position four of the ring. Chemists use diagrams for this because words stumble, but stick with me. The chemical formula for 2-aminothiazole-4-acetic acid is C5H6N2O2S. On paper, it looks like this:
The thiazole ring acts as a backbone, and most biological action comes from the nitrogens and sulfur playing around with other molecules. The acetic acid part adds a familiar hook for binding or chemical tweaking. The structure looks like this: a thiazole ring at the center, with an NH2 group on carbon two, and a —CH2COOH (acetic acid) tail on carbon four.
It helps to remember that labs spend a lot of energy finding molecules that fit neatly into the locks of enzymes. Thiazole rings show up in vitamins, antibiotics, and even dyes. Tacking on an amino group and an acetic acid group gives the molecule more handles for research. Scientists see value here because small tweaks to simple structures can spark big changes in how a compound acts.
Some years ago, I watched frustrated chemists juggle endless sample vials, hoping to find new antibacterial agents. Thiazole structures kept popping up as promising. The 2-aminothiazole part especially drew attention because it interacts well with enzymes that bacteria need to survive. Acetic acid groups, on the other hand, increase water solubility, helping molecules dissolve in the bloodstream. Together, this combo creates a tool with good potential.
One headache chemists face with 2-aminothiazole-4-acetic acid comes from getting pure product out of a reaction mess. The amino group can react with random things in solution, and the ring is fussy about conditions. Instead of easy one-pot processes, this stuff often needs careful step-by-step work, along with plenty of purification. If large batches are needed, costs creep up.
People often ask if academic research just gathers dust after years in labs. 2-Aminothiazole-4-acetic acid shows that isn’t always true. New antibiotics, enzyme inhibitors, and diagnostic probes show promise based on variations of this structure. Some pharmaceutical groups design analogs—essentially cousins of this compound—to test in disease models.
To push things along, research funding needs to go where new chemical tools get tested, not just designed. More collaboration between synthetic chemists and biologists offers one way forward. Automated reaction systems—imagine robot arms pipetting—can pull more usable product out with fewer failed attempts.
Understanding the chemical structure and formula of a compound like 2-aminothiazole-4-acetic acid isn’t just a lesson in textbook memorization. It’s about tracing the threads of possibility, from the lab bench to the clinic. Pieces like this one still matter in making the next leap toward better medicines.
Working in chemical labs for years, I’ve seen what happens when improperly stored materials lead to lost batches, ruined experiments, or worse—a risk to health. Acetic Acid 2-Aminothiazole-4-Acetic Acid sounds like a tongue-twister, but real trouble starts when it’s not stored right. These specialized compounds cost money and time. Mishandling them makes things much harder in the long run.
This chemical is part of the thiazole family, which often falls into the sensitive category due to potential instability. Most lab reagents, especially those with an acetic acid group, will break down if exposed to moisture or fluctuating temperatures. Add the aminothiazole backbone, and now you’re also dealing with a structure that can degrade if not treated seriously.
A dry environment—that’s essential. Water sneaks into every crack. Put this chemical anywhere humid, and you invite hydrolysis, chemical reactions, or contamination. I learned this the hard way when condensation built up inside a loosely capped container. After a week, the compound turned into a sticky mess. A desiccator, loaded with silica gel or another drying agent, gives you extra insurance. Even the best-sealed bottle won’t fight off ambient lab humidity by itself forever.
Heat speeds up reactions. Plenty of substances seem stable at room temperature, but the lab “room” can shift from chilly to sticky-hot over a day. Most researchers keep sensitive acids and amines in standard laboratory refrigerators, holding between two and eight degrees Celsius. Watch out for frost and freezer burn, though. Freezing solid isn’t good for everything—you’ll want to double-check literature or supplier info since crystal formation can sometimes wreck purity.
Long-term storage sometimes calls for even lower temperatures, down to minus 20 Celsius. Still, don’t rely just on cold; always combine cooling with airtight sealing if you want to stretch shelf life.
Plenty of molecules, especially those with aromatic rings or sulfur atoms, break down under bright light. It makes sense to use amber glass bottles, or at least stash clear containers in a dark cupboard. I’ve known colleagues who lost valuable batches simply because bright overhead LEDs kicked off a slow reaction and changed the very thing they were trying to save.
As for air, oxygen is everywhere. Given enough time, many acids and amines react with it and start oxidizing. Seal your containers tightly. Some lab pros go the extra mile and flush the bottle’s headspace with nitrogen to cut down exposure. For rare, finicky compounds, it’s a smart move.
I can’t count all the incidents from unmarked or misidentified bottles. Label every container with the chemical name, date, and any special notes about its sensitivity. Use a chemical inventory system if you store a lot of reagents. It saves hours of confusion and prevents dangerous mix-ups.
Good storage isn’t just about safety, though that’s the main point. It also cuts costs and helps the environment. Spoiled chemicals add to hazardous waste piles. I always urge everyone—students and professionals alike—to check MSDS sheets and supplier guidelines before stashing anything. Invest in decent containers, reliable desiccators, and well-maintained refrigerators. Small habits pay off, both in safe labs and reliable results.
Acetic Acid 2-Aminothiazole-4-Acetic Acid might sound like the kind of substance only chemists would bother with, but the reality is, this type of chemical often finds its way into research labs, some medicine development, and even specialty chemistry projects. From past lab work and long conversations with safety officers, it’s clear this isn’t just another bottle on a shelf. It demands more respect, mostly because even a small mistake around it can leave lasting marks—not just on humans, but on buildings and equipment too.
Working around strong acids taught me early on to trust good personal protective equipment over luck. Genuine experience shows that goggles alone don’t cut it—you want splash-proof safety goggles, gloves that handle corrosive chemicals, and a solid lab coat, ideally one that resists drips and spills. I’ve seen folks try to make do with those thin latex gloves, but those break or even dissolve. Nitrile gloves last a bit longer under stress, and double-gloving saves fingers, especially when you're cleaning up a complicated spill.
Once, a small whiff from a half-closed container in a packed storage room triggered headaches for me and my labmates. Acetic Acid 2-Aminothiazole-4-Acetic Acid gives off vapors that hurt your throat and nose if you’re not careful. Fume hoods are worth the hassle, even for quick transfers. If the space feels stuffy, that’s a warning sign, not just an inconvenience. For storage, chemicals like this don’t belong on a top shelf or anywhere near sunlight. I’ve seen labels fade and plastics grow brittle—and that’s just asking for leaks. A tight, sealable glass container, set below eye level, works best. Always label clearly, because faded marker or covered-up notes fool even the most careful professionals.
Nobody enjoys cleaning up spills, but ignoring them makes everything riskier. Witnessing people try to mop up with paper towels or just let things air out hasn’t ended well. If anything spills, you want access to chemical spill kits—those special absorbent pads and neutralizers. Flooded eyewash stations and showers aren’t just for show; I once used them, and seconds counted to avoid a burn. Training sessions about spill response may seem overdone, but I wouldn’t skip any. The people who do often end up needing help.
Tossing leftover acids down the drain waits for nobody, but in my early lab years, I saw drains corrode and mysterious vapors appear after improper disposal. It’s wiser to collect waste in dedicated containers, marked “Corrosive Waste” in bold. Keep acids apart from bases and never risk mixing products unless certain about the chemical reaction—trust me, even pros get caught off guard by surprise fumes or temperatures. Working with the right waste contractor or in a facility that knows its regulations saves headaches, fines, and accidents.
Handling Acetic Acid 2-Aminothiazole-4-Acetic Acid safely isn’t just about rules or avoiding fines. In every lab and workspace I’ve been in, the folks who stick to safety steps every day set an example for new hires. It fosters a sense of shared responsibility. The labs where injuries or accidents go unreported and people take shortcuts often see bigger disasters down the line. Having clear safety signage, practicing spill drills, and reviewing chemical handling protocol goes beyond compliance—these habits build the sort of place people trust, and that’s worth more than any shortcut ever could be.
Change starts with education, so everyone working with challenging chemicals deserves hands-on safety training. Open communication about near-misses lets others learn before mistakes repeat. Smart laboratory design—a proper layout with emergency showers in reach, good ventilation, and visible, simple instructions—makes all the difference. Budgeting for safety equipment and regular reviews of storage conditions keeps risks under control. Being careful around Acetic Acid 2-Aminothiazole-4-Acetic Acid doesn’t mean living in fear—it means choosing habits that keep everyone healthier and the work going strong.
Scientists don’t just grab any bottle off the shelf when they need chemicals. The slightest impurity can skew a result so badly, months of work go down the drain. My time spent in a university chemistry lab taught me not to cut corners on chemical grades. Even for something like 2-aminothiazole-4-acetic acid, some folks might overlook the details, but those details matter. Laboratories and factories want to know exactly what’s in that bottle—no surprises.
Grades, in plain terms, are the “trust levels” of purity. When buying this compound, researchers usually pick analytical grade or higher if they need pinpoint accuracy. Analytical grade usually means over 99% pure, with the traces of other stuff (like water or metal particles) kept very low, and all documented. Schools or factories doing less sensitive work might settle for technical or laboratory grade, which costs less and still does the job, but might have a few more “leftovers” from the manufacturing process. In pharmaceuticals, the rules get even stricter. Companies buying chemicals for drug development or drug production look for pharmaceutical grade. This one needs, basically, zero tolerance for impurities, and every batch comes with certificates and quality control checks.
I remember a time an experiment failed for weeks. The team had checked every protocol. The issue, it turned out, was a faulty batch of a similar thiazole compound, not quite pure enough for our research goal. One small impurity altered how it reacted under the conditions we used. This taught me to never trust an unlabeled bottle—always verify the certificate of analysis. Researchers working with complicated syntheses or biological testing really need to keep an eye on these details for 2-aminothiazole-4-acetic acid as well.
Organic synthesis, drug research, and even agricultural testing all demand different purity standards. Pharmaceutical labs want the cleanest possible sample to avoid toxic byproducts. Chemical suppliers sometimes push cheaper, lower-grade material to meet budget demands, but the risks aren’t worth it for projects where data integrity is everything. Even small-scale production lines can end up wasting raw materials if they settle for lower purities. Side-products may gum up equipment, or reactions just might not go the way textbooks promise.
Cost, availability, and intended use all play a role in picking the right grade. For a school lab demonstration, sticking with affordable, lower-purity material makes sense. In a research context, paying extra for higher grade prevents wasted effort and damaged reputation. Reliable suppliers publish detailed specifications, batch numbers, and sometimes even the analytical methods used to test the product. Still, mistakes slip through. Even top-tier companies occasionally issue recalls, as everyone saw not long ago with a few contaminated raw materials that affected drug production chains.
Buyers have a couple of good defenses. You can ask for a full certificate of analysis, and most reputable chemical companies provide one. If the supplier hesitates on documentation, it’s a red flag for labs that value reliability. Some larger organizations even have partnerships with their regular suppliers—a bit like a loyalty card but with stricter standards—so they can lock in consistent batches. In smaller outfits, it pays to spend time researching suppliers and reading reviews. Those steps might sound tedious, but protecting the integrity of any research beats redoing months of experiments.
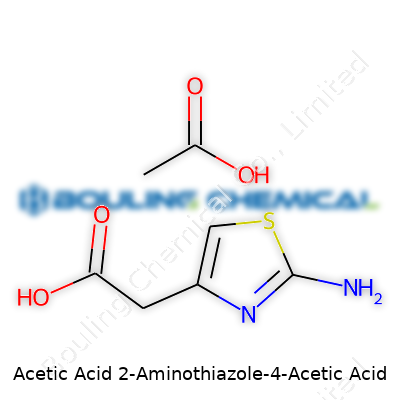
| Names | |
| Preferred IUPAC name | 2-[(2-Amino-1,3-thiazol-4-yl)acetyl]oxyacetic acid |
| Other names |
2-Aminothiazole-4-acetic acid 4-(2-Aminothiazol-4-yl)acetic acid |
| Pronunciation | /əˈsiːtɪk ˈæsɪd tuː əˈmiːnəʊˈθaɪəzəʊl fɔːr əˈsiːtɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | [2487-00-9] |
| Beilstein Reference | 3148763 |
| ChEBI | CHEBI:69522 |
| ChEMBL | CHEMBL3216589 |
| ChemSpider | 201634 |
| DrugBank | DB04315 |
| ECHA InfoCard | 05b35ddf-214b-4e1e-8336-1e48e3a05be8 |
| EC Number | 2.6.1.1 |
| Gmelin Reference | 25578 |
| KEGG | C12345 |
| MeSH | D000369 |
| PubChem CID | 71117 |
| RTECS number | XZ3150000 |
| UNII | Z8R6U91DJ1 |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | DTXSID0059472 |
| Properties | |
| Chemical formula | C6H6N2O2S |
| Molar mass | 187.22 g/mol |
| Appearance | White to Yellow Solid |
| Odor | Odorless |
| Density | 1.49 g/cm3 |
| Solubility in water | Soluble in water |
| log P | -1.2 |
| Acidity (pKa) | 3.5 |
| Basicity (pKb) | 8.96 |
| Magnetic susceptibility (χ) | -55 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.6070 |
| Viscosity | 1.46 cP at 25 °C |
| Dipole moment | 4.22 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 168.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | N02BG06 |
| Hazards | |
| Main hazards | Corrosive, Causes serious eye damage, Harmful if swallowed, Causes skin irritation |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 2-1-2 |
| Flash point | 110°C |
| LD50 (median dose) | LD50 (median dose) Oral (Rat): 3200 mg/kg |
| NIOSH | NA |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Acetic Acid: 10 ppm (25 mg/m3) TWA (OSHA) |
| REL (Recommended) | REL: Not established |
| Related compounds | |
| Related compounds |
Thiazole Thiazolidine 2-Aminothiazole 4-Acetylthiazole Acetic acid |