Long before digital tracking and big pharma patents, amino-substituted pyrrolidines popped up in the lab notes of organic chemists across Europe and the US. The structure seemed simple: a five-membered ring, nitrogen tucked in at one spot, and the kind of amine that draws out both praise and caution. Once scientists nailed down pyrrolidine itself, it did not take decades for folks to realize that introducing an amino group changed interactions, boosted reactivity, and—later on—opened doors in medicinal chemistry. Wartime research, especially from the mid-1900s, gave these small molecules more attention, with a steady uptick as drug discovery pushed into synthetics that mimic or beat nature’s design. By the seventies, several big names in pharmaceuticals started logging patents and publishing research on how different substitutions turned a simple ring into a tool for everything from anti-infectives to enzyme inhibitors.
Aminopyrrolidine usually comes as a crystalline or powdery substance, its form depends on how it’s made and purified. The molecule wears more hats than most basic compounds: it serves as the backbone for antiviral drugs, intermediates for linking up with other functional groups, and templates for fine-tuning receptor activity in research tools. In the lab, it slides between organic synthesis routines, reliable in constructing rings, linkers, and even acting as a chiral auxiliary. Its modest structure belies its versatility, and companies have responded with a spread of catalog items: hydrochloride salts, free bases, protected forms, and custom-ordered derivatives.
What stands out with aminopyrrolidine is its sweet spot of reactivity. The ring is stable under mild conditions, but not so inert that reaction partners ignore it. In the solid state, these compounds often sit as colorless to pale yellow crystals or powders. Most are relatively soluble in alcohols, acetonitrile, and slightly in water—though plenty of variants exist depending on where that substituent sits or what side-chain rides along. Boiling points and melting points stretch across the range, but even the simple parent compound can handle moderate heat. The amine group adds basicity, so the molecule tends to pick up protons in acidic conditions, a trait chemists use to their advantage for easy salt formation.
Labeling for aminopyrrolidine includes more than the molecular formula. Commercial bottles often display structural or IUPAC names, batch numbers, and any chiral purity (if relevant). Typical suppliers specify weight, appearance, melting point, minimum purity by HPLC or GC, and storage recommendations—usually cool, dry, and away from oxidizers. In regulated scenarios, a safety data sheet comes attached, detailing GHS hazard classes and recommended PPE. Labs running GMP or ISO protocols rely on this documentation to track the material from bench to finished product.
Most routes for making aminopyrrolidine root in either cyclization or modification of already-formed pyrrolidine. One classic prep runs through reductive amination: start with a pyrrolidone, tease in ammonia or an amine source, and reduce under hydrogen or using borohydrides. Another tack builds the ring from open-chain amines using condensation and reduction, sometimes via transition metal catalysis. Chemists gravitate toward the method that balances availability of starting materials, by-product control, and necessary scale. Academic labs tinker with green-chemistry tweaks, but industrial processes demand reliability and yield, forcing everything from careful solvent selection to continuous-flow innovation.
Aminopyrrolidine acts like a chemical Swiss army knife once it’s on the bench. The amine loves to react with acyl or sulfonyl chlorides, snapping into amides or sulfonamides. Alkylation happens readily with haloalkanes if a base holds the medium steady. The ring nitrogen survives conditions that might shred open-chain amines, giving medicinal chemists confidence as they load on functional groups. Anyone aiming to make heterocycles finds these compounds work nicely in cyclization reactions, especially for building fused or bridged ring systems. Plenty of research focuses on tweaking the electronics at different positions, shifting pharmacological and physical profiles toward the intended target.
Across catalogs and research papers, aminopyrrolidine wears a wardrobe of names. In the literature, you might spot it as 2-aminopyrrolidine, N-aminopyrrolidine, or even by shorter trade names if the structure gets buried inside a pharmaceutical intermediate. Some protected forms show up as Boc-aminopyrrolidine or Ts-aminopyrrolidine, referencing the groups used to modify its reactivity in synthesis. Pharmacology circles prefer codes like EPC-2 or abbreviation-heavy systematics, though anyone after a CAS number can usually cross-reference easily.
Chemists treat aminopyrrolidine with respect. Amine vapors can irritate, and splashing a concentrated solution burns like any other strong base. Gloves go on before opening bottles, and even in a well-ventilated hood, professionals avoid breathing in dust or mist. If spilled, the material needs cleanup with absorbent pads and bagging for hazardous waste, not the regular trash bin. Disposal rules follow local environmental guidelines, and transport falls under UN hazard classifications. For anyone handling the compound on scale, regular training covers storage, spill response, and emergency procedures. Many companies rotate inventory quickly to avoid shelf-time breakdown.
Big pharma, smaller biotech groups, and academic drug discovery teams all prize aminopyrrolidine. The backbone fits into antiviral drugs, kinase inhibitors, and central nervous system agents. Medicinal chemistry teams build libraries around these rings, mixing and matching substituents to search for hits. Industrial chemists push the molecule toward agrochemicals, tapping its amine for fast conversion. Some material science efforts look at pyrrolidine derivatives for polymer additives or specialty adhesives. The molecule’s adaptability fits a wide range of research and development programs, often moving from bench-top curiosity into clinical trials before most in the public have even heard its name.
The research engine for aminopyrrolidine hums year after year. Every time new viruses crop up or old enemies develop resistance, researchers scramble for ways to tweak established compounds. Many start with aminopyrrolidine, adjusting the scaffold for better binding, improved selectivity, or lower side effects. High-throughput screening has uncovered derivatives with surprising biological activity, and some teams tweak the basic ring for enhanced metabolic stability. Big data and AI, now trending in drug design, often point back toward these simple amines as starting points for analogs. Other researchers explore sustainability by revisiting old syntheses with green solvents or lower energy demands, taking lessons from both bench mishaps and scale-up successes.
No one likes unpleasant surprises, so toxicity studies on aminopyrrolidine started early. While small doses in the test tube show modest cytotoxicity, higher concentrations trigger cell stress—a trait shared by many small amines. Scientists have run rodent studies, tracking metabolism and organ effects, noting risks tied to dose and duration. Chronic exposure or unanticipated metabolites warrant caution, particularly for variants with halogen or nitro substitutions. Industrial hygienists flag aminopyrrolidine for eye and respiratory protection, and waste must stay out of the water table. The toxicology community looks for patterns, warning signs, and safety margins to prevent lab and worker health issues.
The future for aminopyrrolidine and its family is not tapped out yet. Artificial intelligence has started nudging compound design into unfamiliar territory, approving tweaks earlier generations dismissed. The push for new antibiotics and antivirals draws heavy on these motifs, driving partnerships between academia and the private sector. As policy shoves the industry toward cleaner synthesis and sustainable chemistry, researchers revisit old routes and retrofit production with recycled solvents and renewable sources. Diagnostics and targeted therapies may call on these small rings, whether as sensors, imaging agents, or delivery vehicles. For me—and anyone who’s ever spent late nights chasing a mystery peak by HPLC—the humble aminopyrrolidine is proof that foundational chemistry still solves real-world problems, decade after decade.
If you’ve ever picked up a prescription and glanced over the ingredient list, odds are you haven’t seen aminopyrrolidine. It hides in the backbone of some complex drugs, practically invisible unless you have a chemistry background. This compound, part of the pyrrolidine family, deserves a closer look, especially for people following advances in treatment for tough diseases.
Aminopyrrolidine isn’t an end product you buy off the pharmacy shelf. Instead, chemists rely on it as a platform or “building block” that helps craft several high-impact medicines. In my experience poring over pharmaceutical research, I’ve noticed how often this fragment pops up in new treatments, especially for illnesses that don’t respond to simpler therapies.
The first thing that stands out about aminopyrrolidine is its role in drug design. Pharmaceutical companies reach for it because its chemical shape allows them to plug it into bigger molecules without a fuss. This quality becomes a real advantage when tackling complicated medical problems like HIV, certain cancers, or even diabetes.
For example, several HIV protease inhibitors feature this core structure. These drugs force the virus into a tight spot, blocking one of the main enzymes it needs to copy itself. Years ago, treatment for HIV meant a short and rough road. Now, dozens of new drugs—many built using aminopyrrolidine fragments—have turned a once-fatal diagnosis into a manageable condition. Having watched friends get better thanks to these treatments, it’s hard to overstate the significance.
Drug side effects stop more people from staying on their medications than almost anything else. When pharmaceutical scientists use aminopyrrolidine, they can design molecules that aim straight at their target, rather than scattering effects across the body. Some anti-diabetic and cancer drugs with this backbone promise exactly that—precision without the baggage of toxic side effects. That matters to anyone who’s seen a relative struggle with chemo or a friend get frustrated over diabetes drugs that just don’t sit right.
It’s easy to forget, but aminopyrrolidine plays a part in things beyond medicine. Some chemists use it to make agrochemicals—tools that help farmers protect crops. Of course, every time chemistry steps outside the lab and onto farmland, questions about health and environmental safety come up. Watching debates unfold about pesticides and their impacts on bees or water supplies, I keep thinking about the responsibility that comes with bringing any chemical out of the laboratory.
Innovative drugs don’t just appear on the shelf. They grow from pieces like aminopyrrolidine, shaped and reshaped in repeated experiments. In decades past, making a new drug often took years longer and cost far more because researchers didn’t have these convenient building blocks. Now, with molecules like this one at their fingertips, scientists design new drugs faster and with more accuracy—which means treatments land in clinics sooner.
Some of the obstacles remain huge—supply chain gaps, high prices, unexpected effects on people who need these drugs most. Policymakers and scientists need to work together so the right people get access, and that starts by better understanding how these building blocks shape tomorrow’s medications.
Growing up, chemistry classes painted a world where the most unexpected molecules could make headlines. Not every ring-shaped compound grabs attention, but pyrrolidines do it better than most. These five-membered rings, laced with nitrogen, carry loads of potential, and structure always tells a story. Add an amino group, and you get aminopyrrolidine — a building block in drug design, organic synthesis, and research settings.
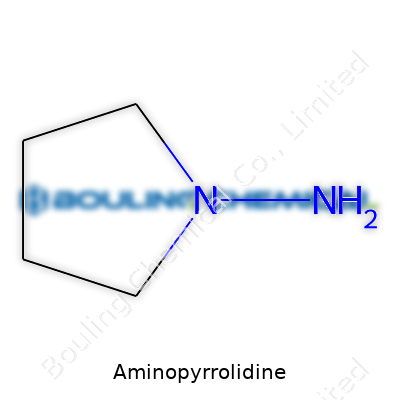
Aminopyrrolidine centers around a simple molecular structure: a five-membered ring called pyrrolidine. One nitrogen atom replaces a carbon in the ring, setting it apart from its cousins. Throw in an extra amino group (-NH2) attached somewhere on that ring, and that’s aminopyrrolidine. For most folks, the most common form means placing the new amino group right on the second spot, so 2-aminopyrrolidine pops up more than you’d expect in papers and labs.
Draw it on paper, and you’ll see a pentagon with a nitrogen node. Tag that extra -NH2 group onto the second carbon, branching right out of the ring. Its chemical formula: C4H10N2. Thanks to those two nitrogen atoms, this small molecule has a punchy reactivity. These nitrogens love to form bonds, especially in the hands of a seasoned synthetic chemist.
Anyone who’s dabbled in pharmacy or biotech research knows molecules like aminopyrrolidine aren’t just abstract diagrams. Several modern drugs lean heavily on this core. It acts almost like a molecular wrench: flexible, sturdy, but ready to lock onto essential reactions. Many antiviral compounds, enzyme inhibitors, and antidepressants build off this backbone.
The presence of both a primary amino group and the nitrogen ring adds basicity, which often helps drugs get absorbed by the body or cross cell membranes. It’s not just theory — studies have shown pyrrolidine-based structures can sneak past cellular defenses, making them handy for medicinal chemists looking to design targeted therapies.
Tinkering with the position of that extra amino group changes everything. Move it from the second carbon to the third, and suddenly, the molecule’s behavior shifts. Sometimes, a single tweak means the difference between treating migraines and triggering unwanted side effects. Research teams wrestle with these details every day. Getting consistent quality and purity remains a challenge, especially for large-scale production.
Safety should never take a back seat. Aminopyrrolidine is relatively simple, but even minor changes can turn a useful intermediate into something toxic or environmentally persistent. Regulatory agencies need to stay sharp, especially as new derivatives hit the market. Labs and companies alike should run regular checks and enforce tight safety protocols, not as an afterthought, but as part of the daily routine.
Instead of trusting tradition, researchers are exploring greener ways to synthesize aminopyrrolidine. Catalysts made from less hazardous materials help cut down on pollution. Automation sorts out impurities, so final products meet high standards without the waste. Open collaboration across university groups, start-ups, and big pharma makes a real difference, spreading better practices further and faster.
Aminopyrrolidine stands as a prime example of how a simple chemical shape can spark big changes. Its structure, easy to sketch but hard to master in practice, keeps finding uses well beyond textbooks. The chemistry community would do well to keep eyes open for both risks and opportunities as science keeps pushing ahead.
Aminopyrrolidine might sound abstract, but lots of specialty labs, pharma teams, and chemical suppliers know it well. This compound pops up in all kinds of research and industry projects, and how it sits on a shelf genuinely makes a world of difference to safety and results. I’ve seen cases where sloppier practices ruined months of effort—simply because someone underestimated proper storage.
Many chemicals catch trouble as soon as rooms warm up or cool down unexpectedly. Aminopyrrolidine fits right into this camp. If you let it get too warm, you start inviting breakdowns and side reactions, which translates to a change in purity and major headaches if you’re chasing accurate data. Most sources agree: keep it cool, somewhere between 2°C and 8°C. That means standard refrigeration, not tossed in a random cabinet. Every time someone forgets the fridge, folks can expect weird color changes, odd odors, or data that just flunks quality control.
I learned early: never underestimate humidity. Leave aminopyrrolidine exposed to air, especially in a place with wet weather or steamy lab conditions, and problems pile up. Water in the air reacts with this compound, sometimes leading to sticky messes or even dangerous byproducts, depending on what else this stuff has seen during use. Dry storage, sealed containers, and desiccant packs make all the difference. Over the years, I’ve watched labs burn through extra budgets fixing problems that came from ignoring this simple trick.
Some compounds shrug off sunlight; aminopyrrolidine doesn’t fall into that bucket. Light exposure can set off breakdown or unwanted reactions. Even a little sunlight can cause subtle changes that later sabotage experiments or production runs. Folks who store bottles in amber glass—with the containers tucked away from windows and harsh lab lights—save themselves unnecessary grief. If the container starts to look yellow or cloudy, someone skipped this step.
I’ve lost count of the times someone grabbed the wrong bottle, mixed it up, and only found out weeks later. Clear, up-to-date labels that include dates, concentrations, and who last handled the compound stop mistakes before they start. No one wants the sort of phone call where a trial falls apart over mislabeling—especially for something that only takes a few seconds to fix.
Aminopyrrolidine, like many research chemicals, doesn’t belong in the wrong hands. It’s not just about child locks; it’s also about tracking who has access, who signs out bottles, and what gets used for which project. Good recordkeeping builds trust between teams, keeps insurance folks happy, and cuts down on mysterious vanishing supplies.
Strong habits make storage part of the lab routine, not something that shows up only after a mishap. Training, spot checks, and an environment where junior team members can ask before guessing keep people—and projects—out of trouble. It isn’t about bureaucracy; it’s about giving everyone a fair shot at safe and productive work.
Plenty of issues fade away with dedicated storage fridges, reliable humidity control, and transparent labeling systems. Add a training session or two every year, and staff begin to look out for each other. I’ve watched labs transform simply by returning to the basics—keeping things cool, dry, sealed, labeled, and secure. It doesn’t sound glamorous, but it’s what sets apart the teams who lead from the ones always in cleanup mode.
Ask folks working in labs about chemicals, and the conversation almost always lands on safety. Aminopyrrolidine might sound like just another mouthful of a name, but understanding its risks carries some weight for anyone using it or living near a place where it's produced.
Lots of people outside the lab probably never hear of aminopyrrolidine. It’s one of those base chemicals used in the making of medicines, pesticides, and specialty materials. Its uses point to a kind of chemical that has to be treated with care. The story here isn’t new—lab folks and production crews dealing with this stuff suit up every day because experience has taught them that handling chemicals casually can come back to bite.
Aminopyrrolidine can irritate skin and eyes quickly after contact. If you’ve ever worked with it, you definitely want gloves and goggles. Breathing it in is no better—vapors may sting lungs and make breathing rough. Researchers have even flagged its potential for causing nausea, dizziness, and headaches after only short exposures. There isn’t much debate on whether or not to use protection; the science and real-world stories show short-term effects are no picnic.
Talking about long-term problems brings out a quieter worry. Studies haven’t yet landed on a full verdict, but there’s reason to proceed with care. Some early work points out the chance for organ damage if exposure climbs, especially when the chemical builds up over time in a workspace with bad ventilation. Chronic chemical exposure has haunted enough industrial workers for people to take repeat exposure seriously now. No one wants an echo of the benzene or lead stories from history, where a slow buildup of harm gets noticed too late.
Policies try to keep up. Today’s workspaces get regular air checks and strict storage rules to make sure vapors don’t concentrate. Labels and warning signs aren’t really optional, and nearly every chemical company offers training on spills and emergency responses. The old pattern of ignoring warnings ended, at least in reputable places, mostly because injured workers or sick neighbors simply cost too much—for everyone. Real-world changes have followed right along with those lessons.
Now let’s talk solutions. Ventilation makes a big difference. Something as old-school as a good hood system cuts the risk to lungs by heaps. Regular health checks for people in high-exposure jobs catch trouble early. Spills aren’t rare, but quick action—a mop-up kit and trained hands—means accidents stay as stories, not disasters. People on the job know the dangers best, so companies that respect field experience usually avoid nasty surprises.
Outside factories and labs, transportation and disposal hold their own risks. Leaks during shipping, accidental dumping, or shoddy disposal at old sites all spell trouble if left unchecked. Community activists have jumped in, pushing for clear labeling, honest reporting, and serious penalties for violations. Local watchdogs keep the spotlight on companies that try to skirt rules.
Handling aminopyrrolidine safely comes down to respect—the mutual kind between worker and employer, between company and community. The real question isn’t whether it’s hazardous or toxic; it’s who pays attention, who cuts corners, and who listens when experience speaks up.
Reading a chemical’s datasheet, you’ll often see a list of “standard” pack sizes that hardly feels useful out in the real world. Lots of us want more than a cut-and-dry list. We want to know what size tubs or bottles actually land in labs, how the people around us store and move this stuff, and why anyone cares if the drum says 25 kg or 200 liters, anyway.
If you’ve spent time in procurement, you see the same pack sizes again and again. Aminopyrrolidine usually shows up in bottles around 100 grams, plastic containers from 500 grams to a kilo, and big drums that go all the way up to 25 kg or even 200 liters for liquid versions. College research labs favor the 100-gram or 500-gram bottles. People running kilo-scale reactions or prepping batches of pharmaceuticals step up to the 5-kg or 25-kg drums. The big drums or carboys tend to show up at plants running multi-ton production. You don’t pop one of those open at a lab bench unless you’re having a very strange day.
Getting the right size means saving time and waste. I once watched a colleague order a 5-kg drum for a semester-long project that burned through maybe 200 grams total. The rest sat in the storeroom, slowly inching toward its expiration date. Nobody likes tossing expensive chemicals or dealing with awkward disposal.
Nobody enjoys dilution or re-bottling potent chemicals—especially when exposure brings health risks. Suppliers get that. Lab managers want something that fits the storage shelf, not a shipping pallet. Plant managers want enough in a drum for a big campaign, not dozens of fiddly bottles. Smaller packs also cut down on the risk of contamination, which hits hard if you’re making reference materials or sensitive drug intermediates.
It’s not all about convenience. Packing also shapes transport costs and safety. For anything even slightly hazardous, the DOT or IATA slaps on limits for what can ride in the public mail, the back of a delivery van, or onboard a plane. People ordering from outside the main industrial hubs might have no choice but to go with the 500-gram or 1-kg bottles, since bigger loads face extra hazmat rules.
Nobody in a busy lab wants bulk containers that need special storage or gear to handle. Small packs mean faster weighing-out, less mess, and, no small thing, easier label checks. Broken containers or spilled powders cost time and morale.
If you don’t see a usable size on a supplier’s catalog, it’s worth calling them. Many offer customized packaging for big orders or recurring customers. I’ve seen colleagues receive compound split into exact aliquots, ready to use, just for the price of a quick phone call. That kind of service stops waste before it starts and keeps both cost and risk down.
People who buy chemicals do well to work closely with suppliers, especially when group needs change. If several researchers need the same compound, splitting orders can curb both costs and leftover stock. Automated ordering linked to project demand also helps, keeping supplies at just the right level.
Above all, being mindful of what’s on hand, what runs out fastest, and where safe handling matters most shapes the pack size decision much more than a standard catalog chart ever could.
| Names | |
| Preferred IUPAC name | 1-Aminopyrrolidine |
| Other names |
(S)-1-(Aminomethyl)-2-pyrrolidinecarboxylic acid Aminopyrrolidine (unspecific) |
| Pronunciation | /ˌæm.ɪ.noʊ.pɪˈroʊ.lɪˌdiːn/ |
| Identifiers | |
| CAS Number | 13475-90-4 |
| Beilstein Reference | **136437** |
| ChEBI | CHEBI:46858 |
| ChEMBL | CHEMBL259367 |
| ChemSpider | 13841062 |
| DrugBank | DB07244 |
| ECHA InfoCard | 14e71c3b-8c60-4859-9231-83c01165bb2e |
| EC Number | 872-90-2 |
| Gmelin Reference | 89337 |
| KEGG | C22110 |
| MeSH | D000648 |
| PubChem CID | 71304 |
| RTECS number | UE8775000 |
| UNII | XYG80G6D1F |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | DTXSID0056422 |
| Properties | |
| Chemical formula | C4H10N2 |
| Molar mass | 114.18 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | D=1.11 g/cm3 |
| Solubility in water | Soluble |
| log P | -0.89 |
| Vapor pressure | 0.0002 mmHg at 25°C |
| Acidity (pKa) | 11.0 |
| Basicity (pKb) | 2.78 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.555 |
| Dipole moment | 2.16 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 317.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -62.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3847.7 kJ/mol |
| Pharmacology | |
| ATC code | N05CM25 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P333+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 61°C |
| Autoignition temperature | Aminopyrrolidine autoignition temperature: 260°C |
| Lethal dose or concentration | LD50 (rat, oral) = 125 mg/kg |
| LD50 (median dose) | 664 mg/kg (rat, oral) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 1 mg/ml DMSO |
| Related compounds | |
| Related compounds |
Aminopyrrolidine derivatives Pyrrolidine Aminopyridine Pyrrolidinecarboxylic acid Diaminopyrrolidine |