Acetyl Morpholine’s story starts in the early twentieth century, at a time when chemists chased new molecules to explore the boundaries of organic synthesis. Folks like Gabriel and Ladenburg dug through morpholine’s reactions, noting how acetylation introduced unique properties. Over the decades, researchers kept tweaking morpholine, documenting acetyl derivatives in German and British patent filings. In the 1970s, industry noticed acetyl morpholine’s clear-cut reactions and stable nature, pulling it from benchwork into applied chemistry. Chemists came to rely on it where amides and morpholine both proved helpful, especially as techniques for amide synthesis improved. Today, chemical manufacturers across Europe, Asia, and North America produce it in tons per year, supplying not just the lab but a spread of industries, each finding value in this compact, versatile compound.
Acetyl Morpholine, as most in chemical circles know, gives a mild amide in a morpholine ring. You find it sold as a colorless to pale yellow liquid, sometimes as crystals when cooled. Chemists keep it on hand for its ability to easily blend into larger syntheses or function as an intermediate in diverse reactions. Several catalogues list it under CAS number 1696-20-4, along with other synonyms such as N-Acetylmorpholine or 4-Methyl-4-oxopiperidine. Companies package it for both research and bulk industrial use, with data sheets highlighting its solvency, reactivity, and low volatility.
Acetyl Morpholine boils around 90-91°C at reduced pressure, and it carries a melting point near -20°C. The molecular weight sits at 129.16 g/mol, built from carbon, hydrogen, nitrogen, and oxygen in a C6H11NO2 formula. Water can dissolve moderate amounts, but it mixes well with ethanol and other polar solvents. On the shelf, it stays stable under ordinary conditions, without any wild reactions or hazards, but strong acids or oxidizers should stay away from it, keeping storage straightforward. If you’ve ever handled it, its faint amine odor is noticeable but never as aggressive as other substituted amides.
Each drum, bottle, or ampul tells its own story, with purity often guaranteed over 98%. Chemical suppliers provide certificates listing NMR, IR, and GC-MS data for each lot. You typically see standard hazard and precautionary statements on the label: "Avoid inhaling vapor," "Use gloves and goggles,"—standard operating table fare for compounds in this family. For global trade, the UN classification ranks acetyl morpholine as low hazard for transport, but compliance with REACH and other local rules is a must for large-scale production, and each label will mention proper storage temperature and shelf life.
Most synthetic routes start by acetylating morpholine directly. Standard ways use acetic anhydride or acetyl chloride in a dry solvent, with temperatures managed under 10°C to rein in exothermicity. The product forms quickly, with water or mild base cleaning up excess reagents. After extraction and drying, rotary evaporation pulls off the solvent, leaving relatively pure acetyl morpholine. Some labs rely on enzymatic acetylation for smaller, greener batches, but big industry sticks to chemical reagents for yield and cost reasons. Purification rarely needs more than one pass through silica or vacuum distillation because the main impurities wash out easily.
Acetyl Morpholine demonstrates enough resilience in most conditions, letting it serve in further amide formation, hydrolysis, and transacetylation. Hydrochloric acid or sodium hydroxide react with it, breaking the acetyl group free in controlled hydrolysis. You can also alkylate or acylate at specific ring positions, making it a springboard for more complex molecules. Its amide group doesn’t push reactions as fast as simple esters, so broader applications include serving as a moderate acyl donor or sometimes a building block for heterocycles in pharmaceutical prep work. Newer research explores its use in metal-catalyzed couplings, taking advantage of the ring structure’s stability.
N-Acetylmorpholine, Morpholine N-acetate, and 4-Acetylmorpholine are common trade names. Researchers running through patent or journal archives might also spot NC 167 or Morpholine, N-acetyl- in older literature. Different markets, especially Asian suppliers, often list product codes alongside the chemical names. This mix of synonyms can trip up newcomers, but most datasheets cross-reference them clearly these days.
Standard chemical hygiene practices cover routine handling. A fume hood and gloves are all you need for most operations, since vapors and splashes can cause mild eye and skin irritation. Chronic exposure hasn’t turned up significant risks in the literature, but it doesn’t mean unprotected work is wise. Spill response involves simple sorbents and ventilation—no special gear required unless fires break out. Waste disposal goes with other mild organic amides. Companies using ton-scale batches will usually file full risk assessments, but everyday researchers rarely run into trouble with this compound.
The compound’s role stretches across several fields. Dye chemists whip it up as a precursor for color additives, taking advantage of the morpholine ring’s stability under harsh processing. In pharma, it acts as an intermediate for active ingredients, rarely as a direct medicine itself but useful in the hands of med-chem professionals. Agrochemical producers sometimes employ it for off-the-shelf reaction building, especially when nitrogen-based rings are on the synthetic path. Notably, it finds favor in specialty polymers as a chain or end-group modifier, imparting thermal or solubility tweaks to the final product line. Each sector looks for a blend of safety, functionality, and ease-of-use, and acetyl morpholine mostly delivers.
University labs and corporate scouts experiment with acetyl morpholine as a scaffold for drug design, especially when aiming for CNS-active molecules, since the ring structure crosses blood-brain barriers well. Catalysis research benefits from its capacity to coordinate light metals, especially in reactions needing mild, non-nucleophilic ligands. In the past decade, green chemistry efforts worked to swap acetic anhydride for bio-based donors, and R&D teams track how each change affects yield and waste production. The search for more effective reaction sequences involving acetyl morpholine continues, aimed at reducing cost, increasing atom efficiency, and simplifying separation.
Lab rodents dosed with acetyl morpholine showed low to moderate acute toxicity, with most tests reporting an LD50 near 2000 mg/kg, oral, in rats. Pure compound causes limited skin and eye irritation; the main threat comes if large quantities reach water due to slow breakdown in aquatic environments. Chronic or reproductive risks remain understudied compared to other morpholine derivatives, but available data suggests occupational exposure should remain low. Standard mutagenicity and genotoxicity tests haven’t raised red flags, but the chemical’s breakdown products under extreme heat can give off more toxic fumes. Industry responses include closed process lines and regular monitoring for vapor release near process vessels. Careful labeling and training make up for the few gaps that still exist in the safety data.
As demand for adaptable, low-footprint chemicals grows, acetyl morpholine stands ready for new roles in specialty synthesis and industrial chemistry. The compound lends itself to next-generation catalysts and battery materials, where ring-nitrogen structures boost performance. Biopolymer research in particular finds promise in morpholine derivatives, as green chemistry pushes for drop-in replacements to harsher synthetic intermediates. Talent shortages in chemical R&D pose a bottleneck, but partnerships between academia and industry keep projects moving forward. More transparent regulatory profiles and open-source safety data will help, so long as production keeps ahead of new rules. In the end, acetyl morpholine looks set to ride the wave of sustainable, multi-use chemistry—it won’t break old conventions, but it will serve where flexibility trumps specialization.
Acetyl morpholine isn’t a household product, but folks working in labs or factories might bump into it without much talk. Most probably wouldn’t even recognize the name, yet its role in making life easier in manufacturing and chemical processes deserves attention.
The place where I first heard of acetyl morpholine was a chemistry lab in college. Surrounded by rows of bottles and mysterious labels, every shelf told a story. Among them, acetyl morpholine caught my eye. Curious as anyone new to the field, I asked my supervisor about its use. He said, “That one helps make things work smoother, literally.” Turns out, he wasn’t joking.
Industries that handle rubber, plastics, and dyes lean hard on chemicals like acetyl morpholine. In the rubber industry, people use it as a plasticizer. That means it helps to soften synthetic rubber, making products feel flexible rather than stiff. Rubber hoses, shoe soles, or seals on appliances all need that give. As we rely more on synthetic materials, demand for chemicals that tweak texture and properties keeps growing.
Think about dye or pigment manufacture. Producers aim to get bright, long-lasting colors that hold up against sunlight, washing, and time. Acetyl morpholine plays a part as a solvent, helping dissolve dyes properly. With better solubility, color spreads evenly and penetrates fabrics more thoroughly. Anyone who’s ever worn a t-shirt that faded too quickly understands the value of keeping colors locked in. Chemicals like acetyl morpholine work in the background to get that done.
Farming and big agriculture might surprise folks here. Seed coatings, crop protection products, and certain fertilizers use blends involving acetyl morpholine. It’s not being grown on the fields, but it quietly supports the technologies that shape reliable harvests. There’s some debate about long-term effects and environmental health, but regulation and constant study keep these questions under active review.
Even the medicine world connects, though not directly in the pills people take. Instead, researchers use acetyl morpholine during drug development to explore new compounds or test reactions. For anyone pursuing a research career, this chemical joins the list of lab companions. It stands as a tool rather than an ingredient.
Given its range of uses, we ought to pay attention to both safety and oversight. Direct handling can irritate skin or eyes, so gloves and good ventilation matter a lot. Industrial waste disposal also deserves focus. Where chemicals end up shapes communities—no one wants polluted water or soil. In my local area, regular workshops teach workers to treat all chemicals with respect, not just the scary-sounding ones. Small measures add up: better training, stricter controls, and open communication between workplaces and local regulators make a difference.
No one chemical makes modern life possible, but acetyl morpholine highlights the hidden effort inside so many ordinary things we handle. A world without it would feel less flexible and more frustrating. That’s why keeping an eye on its journey—from lab benches to factory floors—matters for more than just chemists.
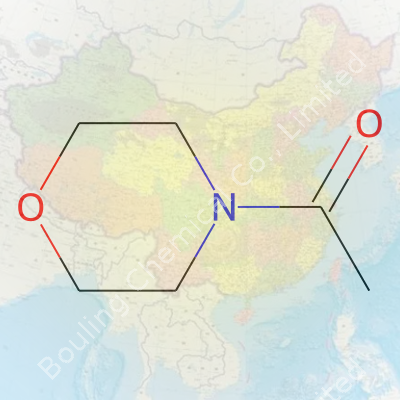
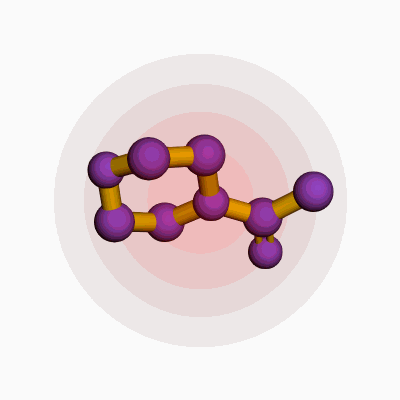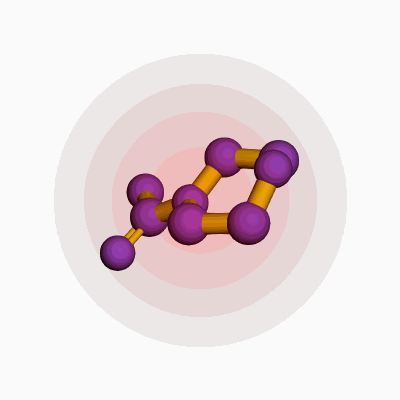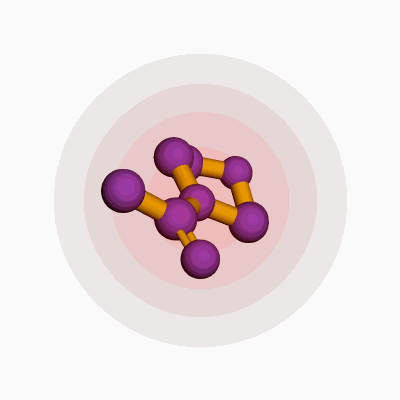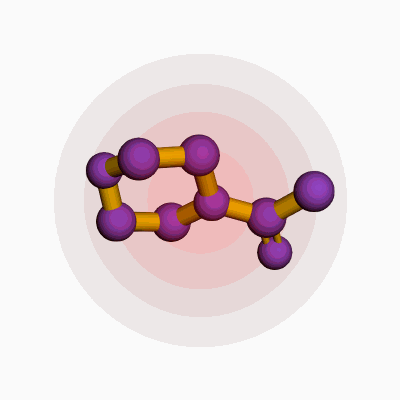
Talk of acetyl morpholine hardly lights up dinner conversation. Despite its modest celebrity, this compound pops up behind the scenes in multiple industries, from pharmaceuticals to specialty chemicals. Chemically, acetyl morpholine’s formula looks like this: C6H11NO2. It mixes a morpholine ring—already a staple in labs the way black coffee fuels night shifts—with an extra acetyl group. Picture its structure as a six-membered ring built from four carbons, one nitrogen, and one oxygen, all easily bonded in a raft-shaped cycle. The acetyl piece, a two-carbon unit snagging a spot on that nitrogen atom, rounds out its profile. Simple at a glance, but with a punch that goes well beyond.
I remember poking through a shelf of reagents, spotting morpholine and its relatives. Morpholine by itself gets love for being easy to dissolve and to take part in all kinds of reactions. Now toss on that acetyl group, and things pick up speed. This small chemical tweak lets it slot into more formulas, kind of like swapping out a guitar string for a new sound. It doesn’t just change “how” the molecule behaves; often, it opens up routes for molecules to be more than the sum of their parts.
Most folks rarely ask why chemists care about structures like acetyl morpholine. Here’s the driver: shape and chemical functionality decide if something works in a process—or if it flops spectacularly. For pharmaceutical makers, the acetyl group can dial down the reactivity of the morpholine base, make it less harsh, or give it new life in making active drug ingredients. In solvents or chemicals meant for manufacturing, slight changes like this can boost safety or shift physical properties in much-needed ways.
Experience has taught me that simple changes, like the acetylation of a ring system, have direct consequences. Imagine working with a raw morpholine: its volatility and reactivity keep you on your toes. Give it an acetyl coat, and suddenly, the material flows differently, stores better, and sometimes sits more comfortably in industrial recipes. These shifts have real weight. For workers handling chemicals, for engineers planning out reaction steps, or for communities looking to reduce environmental risks, structural choices reach far beyond paper models and lectures.
Drawn-out reaction steps and unpredictable behavior bog down chemical supply chains. Talking with friends in specialty chemical plants, the message repeats: reliability wins. Acetyl morpholine stands out for its stability. It resists unwanted side reactions, trims reaction waste, and squeezes profit out of every batch. This reliability helps not only manufacturers but also regulators trying to pin down hazard profiles and emergency responders who need clear facts if there’s a spill.
Thinking back to my own past near-miss with a volatile reagent, the value of predictable, mild chemicals like acetyl morpholine feels real. Safe handling practices come straight from basic chemical structure. Lower volatility, as seen with acetyl morpholine, makes storage and transport less risky. Consistent behavior cuts surprise costs and protects people on the job. If more chemical development keeps these lessons in view, the industry’s daily grind—and its public trust—keeps moving forward.
Acetyl morpholine doesn’t shout for attention, yet it quietly solves problems in both lab and factory settings. Making savvy substitutions with well-understood compounds like this rings true for safer workplaces, more reliable manufacturing, and better outcomes downstream for everyone. Knowledge of chemical structure turns into better real-world choices. Maybe that’s what matters most when complex science meets daily life.
Anyone working around Acetyl Morpholine will want to pay real attention to the way it’s stored and handled. This organic chemical, used in both pharmaceuticals and specialty manufacturing, brings potential hazards that show up if folks get too casual. I once watched a lab tech rush through a small clean-up after a minor spill; he didn’t think much of it at the time, but pretty soon the headaches started. A little knowledge changes the whole approach.
Some stores treat chemicals like a run-of-the-mill cleaning product, but that’s a recipe for mistakes. Acetyl Morpholine should go in a cool, dry, well-ventilated spot. Moisture causes reactions you don’t want to deal with, and hot or sunny corners just speed up any instability in the compound. I’ve seen people try to tuck bottles behind other supplies — that only makes errors more likely. Better to give this stuff a dedicated shelf, away from direct sunlight, far from things like acids, peroxides, and open flames.
Label everything. Over time, faded or missing labels only create confusion. As a young tech, I once reached for what I thought was saline, only to get a bottle of another amine derivative. Simple, clear labeling rules out those kinds of close calls. People think it’s common sense, but it’s only common if everyone sticks to the basics without cutting corners.
Acetyl Morpholine has a knack for causing irritation — skin, lungs, eyes, you name it. Splash it on bare skin, and the burning follows quick. A stray whiff without a mask, and your whole day gets worse. In every lab I’ve worked, folks knew to suit up: nitrile gloves, safety goggles, lab coats, and, if the room gets stuffy, a fume hood or respirator. We don’t look for trouble, so why invite it?
Accidents still happen. Once, a new intern managed to catch a small spill during transfer. He froze, unsure what to grab, wasting precious seconds. I always keep spill kits right by prep areas. This isn’t just for show; quick reaction means less exposure, and less risk for everyone nearby. If it hits your skin, you flush it off — fast and thoroughly.
A lot of people think hazard training once a year covers it all. Real safety culture means refreshing your knowledge, practicing spill responses, and keeping lines of communication open. This isn’t just management’s responsibility, either. I’ve corrected a colleague mid-transfer — no ego, just mutual respect. If one person cuts corners, everyone becomes vulnerable.
Cutting down on open transfers, switching to sealed containers, and automating repetitive dispensing can take most of the risk out of the workplace. Simple investments like secondary containment bins add a layer of protection. Disposal routines matter too; Acetyl Morpholine doesn’t go down the drain or in with the general trash, because it interacts badly with water treatment chemicals, threatening environmental safety.
Underestimating Acetyl Morpholine turns small mistakes into big ones. Working with this chemical demands routines that protect not only the user but everyone who might come near the storage area. Locking down those routines matters more than chasing productivity shortcuts. My own peace of mind relies on these steps being done right — and everyone shares that responsibility, beginner or expert.
Sometimes, you hear a name like acetyl morpholine and it sounds like something out of an advanced chemistry class. But most people working in labs, factories, or warehouses rarely get to sit through that kind of course. Still, anyone handling chemicals in any setting ought to know what's in front of them, especially if there’s a hint of risk. Acetyl morpholine often shows up as a solvent or as a building block for other substances, so it's not some obscure material that never leaves the back shelf. If something is being shipped, stored, or used in decent quantities, people should know where the dangers are.
I’ve come across acetyl morpholine safety sheets more than once at work. Usually, the first question is: will it hurt you, or is it “safe”? If you go by the usual data sheets, this compound can trigger irritation in the eyes and on the skin. Breathing in a lot of acetyl morpholine could give you headaches, dizziness, or a sore throat. It wouldn’t take drinking a whole bottle to get sick. There’s also a risk for the lungs, since vapors can sneak up on you.
There are plenty of nasty chemicals out there with well-known dangers. Take something as familiar as bleach—most folks respect the bottle enough not to mess around. Acetyl morpholine doesn’t pack the punch of, say, formaldehyde or benzene, both known troublemakers. But no one walks around sniffing acetyl morpholine for fun, either. The real concern comes when folks get careless or equipment fails, flooding a space with vapor or spilling liquid on the floor.
A lot of accidents happen because nobody spells out the dangers in plain language. Gloves, goggles, and a mask don’t make you invincible, but skipping them leaves you wide open. Companies sometimes focus on storage or transport, losing track of what workers actually do during a shift. Acetyl morpholine shouldn’t just be locked in a cabinet—a clear label saying “keep off skin and out of lungs” means more to a shift worker than any technical jargon.
Whenever I talk to folks who work around chemicals, most admit they’ve cut corners now and then. That comes from being rushed or just plain tired, not from stupidity. The real fix is making safety routines clear and quick to follow—not so complicated that no one bothers. Handy wash stations and working exhaust fans can stop a small mistake from turning into a hospital visit.
Instead of asking if acetyl morpholine is “toxic,” it makes more sense to ask: “could it harm someone at work?” The answer is yes, if people ignore safety or if the company overlooks good ventilation and protective gear. Clear rules, regular training, and open talk about incidents give everyone a better shot at staying healthy. Recognizing this substance isn’t as famous as some other chemicals shouldn’t mean we ignore the risks.
Acetyl Morpholine isn’t a household staple, but folks in chemical plants know it as a specialty solvent. The truth about its packaging comes down to several points: the needs for safety, the potential risks from leaks, and straightforward handling. In my own years of working next to shipping docks and talking with warehouse crews, I’ve seen how small packaging mistakes can lead to big headaches. With a compound like Acetyl Morpholine, these headaches grow—this is not the kind of stuff you want spilling everywhere.
Suppliers usually go with steel drums for shipping this chemical. Picture blue or gray drums, often with a tight-seal lid. Typical volumes range around 200 liters, which balances bulk transport with manageable lifting. Those working in the lab or with small-scale applications may see metal cans or bottles—mostly one to five liters—sometimes glass, but usually lined metal so things don’t corrode or react.
Every time I see one of these containers arrive, there’s a checklist process: Are the seals tight? Are labels clear? Is there any sign of corrosion or leaks? In big facilities, damaged packaging leads to downtime or, far worse, an emergency cleanup.
Acetyl Morpholine doesn’t show up packed in sacks or big bags often. That’s mostly because it absorbs water and loves to react when it gets the chance. Chemical drums hold up better to bad weather, rough shipping, and the odd forklift mistake. Producers don’t want to risk an entire load going bad from moisture sneaking in.
Some compounds can handle plastic drums, but this chemical bites into plastic over time. Stainless steel and lined vessels survive repeated use. Suppliers stick to what lasts on the road and in the warehouse—it’s rare to see shortcuts here, because nobody’s interested in a thousand-dollar spill or a lost batch.
Trust in packaging comes down to two things: can the outside tell you what's inside, and will the container stay sealed? Regulations require clear labeling for chemicals. I remember opening a drum once without visible labeling—everyone stopped what they were doing until the foreman sorted out what it was. It wasted a half hour and left everyone uneasy.
Shipping companies look for hazard diamonds and readable handling instructions; rescue gear is often kept nearby if something goes wrong. This is daily routine on any chemical floor.
Every routine shipment still hides potential hazards. I’ve seen companies start using digitally tracked seals—scan a QR code, log the chain of custody, prove it never got tampered with. More operations invest in anti-leak spouts or vent caps. Simple fixes (even reinforced rings on steel drums) keep the risk of worker injury low and save big on cleanup later.
The lesson sticks with me: packaging for chemicals isn’t about cutting corners or chasing the newest container fad. It's about what works: tight seals, chemical-compatible material, clear information, and respect for the people doing the shipping and unpacking. Anyone relying on shortcuts quickly learns the real cost when a dangerous chemical gets out of its box.
| Names | |
| Preferred IUPAC name | 1-(Morpholin-4-yl)ethan-1-one |
| Other names |
1-Acetylmorpholine N-Acetylmorpholine Morpholine, 1-acetyl- Morpholine, N-acetyl- |
| Pronunciation | /əˈsiːtɪl ˈmɔːrfəˌliːn/ |
| Identifiers | |
| CAS Number | 1696-20-4 |
| Beilstein Reference | 1209248 |
| ChEBI | CHEBI:131450 |
| ChEMBL | CHEMBL19670 |
| ChemSpider | 26709 |
| DrugBank | DB08419 |
| ECHA InfoCard | 100.011.070 |
| EC Number | 212-519-4 |
| Gmelin Reference | 1231323 |
| KEGG | C02484 |
| MeSH | D000093 |
| PubChem CID | 15608 |
| RTECS number | SE0175000 |
| UNII | 5B1X6U50FG |
| UN number | 2810 |
| CompTox Dashboard (EPA) | DTXSID1027663 |
| Properties | |
| Chemical formula | C6H11NO2 |
| Molar mass | 143.18 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | fruity |
| Density | 1.073 g/mL at 25 °C (lit.) |
| Solubility in water | slightly soluble |
| log P | 0.02 |
| Vapor pressure | 0.03 mmHg (25°C) |
| Acidity (pKa) | 8.35 |
| Basicity (pKb) | 6.38 |
| Magnetic susceptibility (χ) | -57.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.456 |
| Viscosity | 30 mPa·s (25°C) |
| Dipole moment | 4.03 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 230.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -522.65 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3654 kJ/mol |
| Pharmacology | |
| ATC code | N02AA14 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: H302, H315, H319 |
| Precautionary statements | Precautionary statements: P261, P305+P351+P338, P405, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | Flash point: 98°C |
| Autoignition temperature | 260°C |
| Explosive limits | Explosive limits: 1.72–11.5% |
| Lethal dose or concentration | LD50 (oral, rat): 2150 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1200 mg/kg (rat, oral) |
| NIOSH | NO DATA |
| PEL (Permissible) | Not established |
| REL (Recommended) | 12-24 Months |
| Related compounds | |
| Related compounds |
Morpholine N-Methylmorpholine N-Ethylmorpholine 2-Ethylmorpholine Piperidine Acetylpiperidine |