Roots of 1-benzothiophene chemistry run deep, stretching across decades of steady progress in sulfur-containing heterocycle synthesis. The journey toward 7-chloro-3-methyl-1-benzothiophene began with early forays into benzothiophene ring construction in the early 20th century. Chemists pieced apart the aromatic sulfur fragment, searching for more selective methods. The real shift came with better halogenation techniques in the 1960s and streamlined Friedel-Crafts methylations. As pharmaceutical and agrochemical industries ramped up in the 1980s, demand for substituted benzothiophenes soared. R&D budgets targeted cleaner routes, less waste, and higher selectivity, leading to the robust processes in use today. This compound's historical arc tells a story of persistent refinement, as technology evolved and priorities shifted toward greener, safer, and more scalable synthetic methods.
7-Chloro-3-methyl-1-benzothiophene acts as a solid building block for research and industry. Its unique framework brings together a methyl and a chloro group across a fused aromatic sulfur-containing ring. Manufacturers supply it for advanced synthesis, drug discovery, and crop protection research. The compound's structure — both rigid and reactive — offers distinct opportunities for further transformation. Researchers turn to it for scaffold hopping, seeking new routes to anti-inflammatory drugs or specialty agrochemicals. In comparison to its unsubstituted cousins, the electron-rich sulfur and halogen substitution change its chemical personality. Folks working in high-throughput chemistry value ready access to it through reliable vendors, and a steady global market has sprung up to meet those needs.
7-Chloro-3-methyl-1-benzothiophene typically appears as a crystalline white or pale yellow powder. Its melting point usually hovers between 60°C and 90°C, depending on purity and small synthetic differences. The compound's aromatic nature gives it limited solubility in water, but it dissolves easily in common organic solvents like dichloromethane, chloroform, and ethyl acetate. Chemical stability ranks high, though strong acids or bases attack its ring over time. The chlorine atom draws electron density, making the ring system less prone to oxidation. This simple combo—aromatic ring, sulfur, methyl, and chlorine—balances reactivity with shelf-stability, so long as it's kept out of strong UV light or overly moist conditions.
Vendors typically provide specifications that point out purity above 97%, with NMR and HPLC data supporting the claim. Color, appearance, and melting range appear right on the label. Storage recommendations focus on tight-sealed vials under dry, cool air. Labeling includes the full chemical name, batch number, date of synthesis, and hazard warnings, as per international requirements. Safety sheets spell out hazards for skin, eyes, and respiratory exposure even in trace amounts. Delivery channels favor glass or fluoropolymer bottles, preventing leaching or unwanted reactions. Clarity in technical documentation stacks up as an industry norm, both for regulatory compliance and for the comfort of users demanding traceability from bench to finished product.
Synthesizing 7-chloro-3-methyl-1-benzothiophene presents both challenge and craft. Most labs start with 3-methyl-1-benzothiophene, targeting the seventh position for electrophilic chlorination. Typical routes go with N-chlorosuccinimide or chlorine gas in acetic acid. Temperature control matters, as poor monitoring brings side products and tarry overload. Variants may introduce the methyl group via Friedel-Crafts alkylation of 1-benzothiophene, using methyl chloride in the presence of aluminum chloride, prior to selective halogenation. More advanced green chemistry weighs in, swapping out traditional chlorination for solid-supported reagents or photochemical methods that cut environmental impact. As technology continues to develop, scale-up efforts press for less hazardous waste and easier purification schemes.
Organic chemists recognize how 7-chloro-3-methyl-1-benzothiophene behaves under a variety of reaction conditions. The methyl group at the third position resists oxidation and reduction in mild settings but undergoes bromination or amination with more assertive reagents. The chlorine at the seventh spot offers a site for Suzuki coupling, nucleophilic substitution, or palladium-catalyzed cross-coupling, giving access to virtually any aryl, alkyl, or heterocyclic adduct. Its sulfur-containing core withstands gentle conditions but succumbs to strong acids or radical reactions. This raises the versatility—chemists use it in multi-step syntheses to make targets with varied bioactivity. The molecule adapts, serving as either a parent compound or an intermediate for more complex and functionally diverse structures.
While “7-chloro-3-methyl-1-benzothiophene” remains the most precise, catalogs and patents toss up other names. Some labels go with “3-methyl-7-chlorobenzothiophene” or the systematic “7-chloro-3-methyl-1-benzothiophene.” Others reference trade names which shift depending on region or vendor. Standardization in naming proves critical for avoiding confusion and duplicate orders, particularly when researchers operate globally, share samples, or source from multiple suppliers. Cross-referencing every synonym in spectral libraries and regulatory filings ensures experiments move smoothly, without chemical mix-ups or troublesome paperwork delays.
Working safely with 7-chloro-3-methyl-1-benzothiophene calls for good habits and sharp oversight. The chlorine atom signals caution for skin and lung exposure, while sulfur rings in aromatic settings sometimes create potent odors or secondary irritants when heated. Labs provide fume hoods, gloves, and PPE as standard gear. Storage away from heat or open flames prevents unintended volatilization or decomposition. Disposal routes must follow national and international codes, as halogenated waste brings tighter scrutiny. Training programs for safe handling aren’t optional; a simple misstep can cause lasting injury. I’ve seen researchers who skip gloves because “it’s just one quick transfer,” only to end up with rashes or worse. Frequent safety drills and up-to-date MSDS files keep everyone in the loop, reinforcing the message that every routine counts.
The breadth of uses for 7-chloro-3-methyl-1-benzothiophene stands out. Medicinal chemists tap into its chimeric ring system in the hunt for anti-inflammatory, antifungal, or even anticancer compounds. The aromatic sulfur scaffold resists degradation, translating well into molecules that last longer in biological systems without rapid breakdown. Agrochemical researchers chase new fungicides or pesticides based on its substitution pattern, aiming to outsmart rising resistance. Material scientists also dig into its pi-rich backbone, adapting it as a seed for semiconductors or organic light-emitting diodes (OLEDs). In my experience, the real magic happens when cross-disciplinary work begins: one lab tweaks the molecule for bioactivity, another retools it for electronic usefulness. Each breakthrough paves a path for applications beyond the boundaries of a single field.
Investment in R&D around this compound hasn’t slowed. Drug discovery teams mine its framework for unexplored analogs, manipulating the chlorine or methyl moiety in search of stronger activity or lower toxicity. The core ring structure often serves as a “privileged scaffold,” a chemical platform recognized for generating good leads against tough biological targets. In my previous collaborations, whole libraries of derivatives filled shelves, each one a minor tweak that might produce a major effect. On the process side, greener syntheses cut emissions and protect workers, all while trimming costs. Pushes for in situ monitoring, smaller reactor footprints, and continuous flow manufacture align with 21st-century pressures to deliver both safety and speed. Each dollar spent on smarter chemistry opens new markets and addresses problems like resistance or rare side effects.
Understanding the toxicity of 7-chloro-3-methyl-1-benzothiophene trails closely behind its development. Modern screens probe its effect across cell cultures, rodents, and aquatic species. Chlorinated aromatics often raise a red flag, and long-term testing stretches over months or years to catch delayed chronic effects. So far, acute toxicity registers as moderate: high doses cause irritation, mild liver effects, or sedation in animal models, though it lacks the severe poison profile of simpler halobenzenes. Ecotoxicology occupies a growing slice of attention, with bioaccumulation potential scrutinized in soil and water. Each experiment adds data points that regulators use to set maximum permissible levels, labeling requirements, and disposal recommendations. Transparency matters; publishing negative or cautious results means downstream developers can avoid old mistakes.
Looking forward, 7-chloro-3-methyl-1-benzothiophene remains poised for an expanded role in both core chemistry and real-world products. Markets for designer drugs and advanced crop protectants continue to value its adaptable ring system. Improvements in green chemistry—think recyclable catalysts, solvent-free conditions, or AI-driven process optimization—promise cleaner, cheaper production routes. As regulations tighten on traditional pesticides and pharmaceutical intermediates, the push for lower toxicity and smaller environmental footprints grows louder. Innovations in electronics throw in additional demand for sulfur-rich, high-performance organics with tunable electronic properties, and this compound’s backbone checks several boxes. As I see it, cross-sector partnerships will unlock unexpected uses, extending its influence beyond today’s labs and fields to tomorrow’s medicine, agriculture, and advanced materials.
Looking at 7-Chloro-3-Methyl-1-Benzothiophene brings up memories of undergraduate organic chemistry, staring at skeletal formulas and wondering how a few tweaks could spin a whole new molecule. Imagine benzothiophene: it's a bicyclic ring system with sulfur replacing a carbon in a benzene ring, which sets a foundation for many pharmaceuticals and agrochemicals. In the case of this specific compound, attach a chlorine atom at the seventh position, and stick a methyl group onto position three; the result is a molecule with very distinct chemical properties.
Swapping a hydrogen for a chlorine atom might look minor on paper, but this change brings real-world impacts. Chlorine isn’t just bulkier than hydrogen. Its electronegativity pulls electrons, shifting how the molecule behaves in a synthetic reaction or inside a biological system. The methyl group brings more than just extra mass; it crowds the ring, changing how the entire structure fits with enzymes or catalysts.
From personal experience in an academic lab, I saw how small edits like these meant the difference between a dead-end and a breakthrough. For benzothiophenes, a chlorine in the right spot blocks some reactions, but also paves the way for more targeted modifications used in drug synthesis.
Synthetic chemists see molecules like 7-Chloro-3-Methyl-1-Benzothiophene as a backbone for new therapeutics. For instance, benzothiophene derivatives show up in anti-inflammatory and anticancer research, as well as crop protection. The trick is finding a structure that balances potency with safety. Adding a methyl or chlorine can make a molecule less vulnerable to metabolism—but sometimes, this leads to environmental persistence. The same stability shaping a good medicine might cause a pollutant to linger in water or soil.
A study out of Japan demonstrated that benzothiophene derivatives, especially ones with halogen atoms like chlorine, resist natural biodegradation more than their non-halogenated counterparts. Chemists working on greener synthesis routes keep this in mind, developing molecules designed to break down more predictably.
Reducing the environmental impact of halogenated aromatics starts in the lab. Choosing greener solvents, using mild reaction conditions, and screening new molecules for not just efficacy but also their environmental footprint make a huge difference. Pharmaceutical companies often run soil and water simulations to guess how molecules degrade after use—not just assuming they vanish after a pill gets swallowed or a pesticide is sprayed.
7-Chloro-3-Methyl-1-Benzothiophene shows how chemistry influences lives outside the lab. Those chlorine and methyl groups aren’t just decorations on a ring—they decide if a chemical helps, harms, or hangs around. Molecular tweaks should always involve a look at the bigger picture, whether for a new medicine, a new plasticizer, or even a classroom experiment.
These chemical structures are more than lines and labels. They hold power over health, the environment, and innovation. Chemists and engineers do their best work acknowledging not just the science, but also the responsibility: every bond drawn or atom swapped could ripple much further than the laboratory notebook suggests.
The average person probably never hears about compounds like 7-Chloro-3-Methyl-1-Benzothiophene unless they’re buried deep in a pharmaceutical patent. Still, this molecule plays a bigger role in medicine and industry than most folks realize. Sitting at the intersection of chemistry and health, this compound carries plenty of weight in labs and manufacturing plants.
Drug development draws a lot from molecules like 7-Chloro-3-Methyl-1-Benzothiophene. Its structural backbone offers flexibility, which lets research chemists build molecules that target human diseases with precision. The chloro and methyl groups bring just enough difference in its core, making it a solid building block for tweaking activity in early-stage compound screening. At places I’ve worked, early phase drug discovery often leans on these types of core fragments. We try to block, fine-tune, or stimulate different biochemical pathways by changing just a few atoms in the base molecule. This compound, because of its chemical properties, shows up in projects focused on anti-inflammatory, neurological, or even oncological therapies.
There’s a long list of benzothiophene-derived drugs making waves, particularly in treatments for osteoporosis and certain cancer types. Raloxifene, for example, is built on a similar ring system. The reason chemists come back to these rings? They tend to stick around in the body just long enough to get things done without causing mayhem elsewhere.
Looking past pharmaceuticals, this molecule finds work in material science. Dye and pigment industries use benzothiophene cores to create colors that last—or that behave differently under certain lights. The chlorine modification means fibers or plastics dyed with these derivatives often resist fading better in sunlight. The methyl group further changes how the molecule interacts with different substrates, which can improve adhesion and color fastness.
Working on sustainable coloring agents, it’s hard to ignore how minor tweaks to a molecule can mean less dye running off into our water systems. Smaller, sturdier dye molecules built from benzothiophene platforms reduce waste, and that’s something textile engineers are paying attention to as climate concerns deepen.
It’s not all high science, either. Agrochemical designers examine this compound when seeking new herbicides or fungicides. In plant protection, tweaks to a molecule’s core can change how it attacks pests, or how quickly it breaks down in the soil. The sulfur and chlorine atoms shift the molecule’s electron density, which helps it slip past some resistance mechanisms in weeds or fungi. This opens new options for farmers who struggle with pests that have learned to dodge older chemicals.
Industrial chemistry leans on similar building blocks when working on lubricants, corrosion inhibitors, and even in certain polymers. From what I’ve seen, if a chemical works for one tough job, someone’s already testing it for another challenge somewhere else.
As a researcher, I’ve learned that each application comes with a dose of responsibility. Chemical companies face pressure—both from regulation and the public—to keep products as safe as possible. With molecules containing chlorine and sulfur, the stakes get higher. Waste disposal, worker protection, and environmental impact all become topics in regulatory audits.
Solving these challenges calls for better engineering, transparent supply chains, and more accessible toxicology data. Teams that provide updated safety sheets, invest in waste treatment, and adapt to emerging research help ensure these chemicals bring value without trading off public health or the environment. Chemical innovation doesn’t happen in isolation. It’s shaped by laws, market needs, and a growing demand for full accountability.
If you work in a lab or factory and handle chemicals, 7-Chloro-3-Methyl-1-Benzothiophene probably isn't just another name on a bottle. This compound, like many organic chemicals, can cause trouble if you don’t take care. Researchers from my graduate group went through safety briefings before each project, and not once did they regret those extra steps after a spill or near-miss. This compound can irritate eyes, skin, and—if you’re not careful—may cause breathing problems. No need to panic, but respect keeps you safe.
Gloves aren’t just for show. I remember one lab mate who skipped nitrile gloves, thinking a quick task didn’t matter. She felt tingling within minutes. Nitrile or neoprene gloves stop most organic compounds from getting to your skin, so there’s no reason to risk it. Safety specs block splashes. A lab coat shields your arms and keeps your clothes clean—and with compounds that can stain or irritate, one less headache. Closed shoes and long pants protect your feet when bottles slip or splash.
Open benches in my old school’s labs collected plenty of dust, but nothing beats proper hoods for any chemical that gives off fumes. Fume hoods move vapors away from your nose and lungs. Without one, no amount of holding your breath stops inhalation. If you work outside a big lab, at least make sure you’ve got exhaust fans running. Windows may help, but moving air away from your face matters more.
Washing up doesn’t end after high school chemistry. After handling any organic compound, get to a sink and clean your hands well with soap and water. I remember classmates who skipped this step, then ate lunch and ended up with stomach upset. People forget how quickly chemicals transfer from doorknobs, pens, or phones. Ditching gloves right also matters—if you pull them off wrong, you risk touching the outside anyway.
If you need to keep 7-Chloro-3-Methyl-1-Benzothiophene around, store it in sealed glass containers. Strong acids or bases nearby raise explosion or fire risks, so give each compound space. Labels with proper chemical names, hazard warnings, and dates keep everyone safe. Old bottles without labels in my old lab always raised alarms. Better to over-label than forget what’s inside.
A bottle dropped on the floor sends people running. Don’t rush, but don’t wait. Absorb spills with pads or vermiculite, then put waste in special disposal containers. Regular paper towels just spread the mess. Open the doors or windows, keep people back, and never sweep dry powder—sweeping raises dust into the air. Everyone in the lab should know where spill kits are and how to use them—one person’s quick action can protect the whole team.
Safety data sheets actually help, even though they can read like legal jargon. They list fire hazards, what to do if someone gets a splash, and how to clean up. When rules change or new information comes out, don’t ignore it. Ongoing training, combined with good signage and peer reminders, saves hassle and health. Shortcuts make for quick mistakes, and no chemical is worth an emergency room visit.
Over the years, science has built a way to simplify and organize chemical data. The Chemical Abstracts Service (CAS) number helps cut through confusion. By using a unique numerical identifier, labs, suppliers, hospitals, and regulatory bodies can talk about chemicals without tripping on slang or initials. This means anyone across the globe, whether a research student in Mumbai or an environmental chemist in Berlin, can figure out precisely which substance is at hand.
In my own time working in a shared research lab, I ran into molecules with names like 7-Chloro-3-Methyl-1-Benzothiophene. It’s a chemical that sounds a bit intimidating, but the CAS number, 18841-25-7, brings clarity. No need to wrestle with similar-sounding compound names or gamble on translations—this one number carries the whole identity.
Mix-ups in chemical supply orders happen more than folks like to admit. A few years ago, a colleague ordered a compound for a reaction but ended up with a close cousin because the supplier missed a digit in the code. The experiment failed, time went down the drain, and it cost the department real money. Mistakes like these fuel the push for accuracy. The CAS number serves as that touchstone for product verification, which in turn promotes safe laboratory practice.
Chemicals like 7-Chloro-3-Methyl-1-Benzothiophene don’t turn up only in universities or pharmaceutical labs. These compounds enter environmental monitoring and even crop up in the patent landscape. Those handling them rely on CAS numbers to sift through regulatory demands—think international shipping documents, safety data sheets, or toxicology reports. All these systems depend on fast, unquestionable identification of each substance.
Manufacturers, traders, and customs agencies want a common language. The market for fine chemicals has gone global, so the need for a universal identifier keeps growing. I’ve watched small startups struggle to get their products into Europe because of labeling errors. A single mistake in documentation can send whole shipments back at the border, leaving companies eating heavy losses. Without precise tagging—like marking 7-Chloro-3-Methyl-1-Benzothiophene as 18841-25-7—inventories can turn into expensive mysteries.
Education holds a key role in tamping down identification mistakes. Training in recognizing and accurately reporting CAS numbers should start early, not just during graduate work. Laboratories and suppliers need robust systems that double-check entries at every handoff. Barcoding technology, digital order forms, and even AI-driven cataloging promise to chip away at hazards of human error.
Global organizations such as the World Health Organization and chemical safety boards encourage harmonization. They keep working on databases where CAS numbers anchor checks on toxicity, environmental impact, and handling—resources just about anyone can tap into. In an age of rapid scientific growth and worldwide supply chains, such standards guard against accident and fraud.
Whether making new medicines or certifying industrial shipments, trust comes from getting the details right. In the end, 7-Chloro-3-Methyl-1-Benzothiophene may only be a line in a database for most people, but its CAS number 18841-25-7 forms a backbone that helps move science forward. Clear labeling, robust training, and reliable verification tools don’t just help companies—they protect health, keep work honest, and set the stage for new discoveries.
Storage and disposal of specialty chemicals isn’t just a checklist for labs. It’s about real people, safe workplaces, and the world downstream from you. Years in laboratory environments and conversations with colleagues have shown me that taking shortcuts with chemicals such as 7-Chloro-3-Methyl-1-Benzothiophene leads to accidents and contamination that never end at the point of misuse. This compound—a benzothiophene derivative—brings with it a set of hazards that demand respect, not just routine handling.
7-Chloro-3-Methyl-1-Benzothiophene isn’t designed for a shelf next to snacks. Its chemical nature makes it sensitive to heat, light, and incompatible substances. Organic compounds containing halogens can release toxic gases or break down unpredictably if combined with the wrong materials or stored above room temperatures. Years ago, I saw what happens when incompatible solvents cluster together—corroded shelving, fumes in the air, other containers swelling dangerously.
Best practice means using a tight-sealing, chemical-resistant container, like one made from amber glass or high-grade polyethylene. Keep this container in a cool, dedicated flammables cabinet, far from oxidizers and sources of ignition. Stack bottles by size and hazard level, not in random rows—one spill or knock can trigger chain reactions. Regular visual inspections catch leaks or crystal buildup early. If your site logs chemical lot numbers and opening dates, traceability helps pinpoint issues before a small leak grows into a clean-up nightmare.
Disposing of benzothiophenes takes more than pouring down a drain or throwing in the trash. Chemicals that include halogens or sulfur wreak havoc in soil and water, poisoning fish, wildlife, and eventually, people sourcing water downstream. Environmental agencies closely watch for pollutants from such compounds because even parts per billion create toxic exposures.
A licensed hazardous waste provider should handle every drop, every contaminated glove, and all rinsate. In many jurisdictions, strict chain-of-custody logs and disposal manifests are mandatory. Take those seriously—one lost record can cost your organization six figures, not to mention harm to local communities. If you’re in a smaller facility unsure of protocols, contact your local environmental safety office or university lab safety team. My own experience tells me that building a good relationship with waste haulers and regulators saves money and stress down the line.
Most chemical safety failures happen because someone underestimated a risk or never read an updated safety data sheet. Training staff isn’t a yearly checkbox. I’ve found value in hands-on demonstrations and peer coaching sessions—let people see reactions, not just slide shows. Keep physical and digital copies of SDSs handy, and encourage reporting of every near-miss. If your equipment budget allows, supply spill kits and personal protective equipment right at the point of use.
The answer isn’t just labeling some cabinets and making people wear gloves. It’s fostering a culture where safety is integrated into routine work: clear procedures posted near storage areas, regular audits by people actually using the chemicals, support from management when someone flags a storage or disposal risk. By treating 7-Chloro-3-Methyl-1-Benzothiophene with the caution it earns, you protect more than your current project—you preserve health, reputation, and the environment for all who come after.
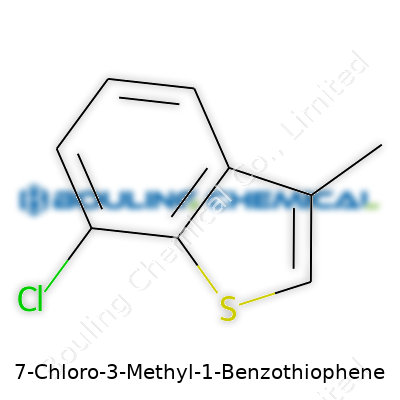
| Names | |
| Preferred IUPAC name | 7-chloro-3-methyl-1-benzothiophene |
| Other names |
7-Chloro-3-methylbenzo[b]thiophene 3-Methyl-7-chlorobenzothiophene 7-Chloro-3-methyl-1-benzothiophene 7-Chloro-3-methylbenzo[b]thiofene |
| Pronunciation | /ˈsɛvən-ˈklɔː.rəʊ-ˈθriː-ˈmɛθ.ɪl-ˈwʌn-bɛn.zəʊˈθaɪ.iːn/ |
| Identifiers | |
| CAS Number | [16525-42-9] |
| 3D model (JSmol) | `7-Chloro-3-Methyl-1-Benzothiophene; JSmol 3D model string: C1=CC2=C(C=C1Cl)SC=C2C` |
| Beilstein Reference | 2089880 |
| ChEBI | CHEBI:140449 |
| ChEMBL | CHEMBL521391 |
| ChemSpider | 126973 |
| DrugBank | DB08399 |
| ECHA InfoCard | ECHA InfoCard: 100.029.884 |
| Gmelin Reference | 108086 |
| KEGG | C14345 |
| MeSH | D029423 |
| PubChem CID | 164927 |
| RTECS number | GL9375000 |
| UNII | WF25O09M44 |
| UN number | UN3437 |
| Properties | |
| Chemical formula | C9H7ClS |
| Molar mass | 192.68 g/mol |
| Appearance | White to yellow solid |
| Odor | Characteristic |
| Density | 1.283 g/cm3 |
| Solubility in water | Insoluble in water |
| log P | 3.97 |
| Vapor pressure | 4.3E-3 hPa (25°C) |
| Acidity (pKa) | pKa = 23.1 |
| Basicity (pKb) | 7-Chloro-3-Methyl-1-Benzothiophene" does not have a reported pKb value, as it is not a basic compound and lacks a basic nitrogen atom. |
| Magnetic susceptibility (χ) | Magnetic susceptibility (χ) of 7-Chloro-3-Methyl-1-Benzothiophene is -74.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.6930 |
| Dipole moment | 3.51 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 340.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 193.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -5729.6 kJ/mol |
| Pharmacology | |
| ATC code | N05CF04 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P332+P313, P337+P313, P362+P364, P501 |
| Flash point | 67°C |
| Lethal dose or concentration | LD50 Oral Rat 3700 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: >5000 mg/kg |
| NIOSH | PA9325000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
3-Methyl-1-benzothiophene 7-Chloro-1-benzothiophene 1-Benzothiophene 7-Bromo-3-methyl-1-benzothiophene 3-Methylthio-1-benzothiophene |