The journey of 7,8-Dihydroxy-2-Phenazine Sulfonic Acid goes back to the early days of heterocyclic chemistry, a time when chemists hunted for new dyes and functional molecules to serve medicine and technology. Early research on phenazine compounds revealed bright color and strong chemical personality, setting them apart from more pedestrian molecules. As sulfonation techniques matured in the first half of the 20th century, researchers leveraged the power of sulfonic acid groups to boost solubility in water, an advantage missing from many classic phenazine dyes. Over the decades, the focus shifted toward analytical applications and redox chemistry, as phenazine derivatives began showing utility in biological staining, electronic materials, and bioactive compounds. My own entry into the lab world introduced me to phenazine sulfonic acids as robust candidates for everything from electron shuttles to potential antibiotic scaffolds, showing how chemical traditions evolve while building on the shoulders of foundational discoveries.
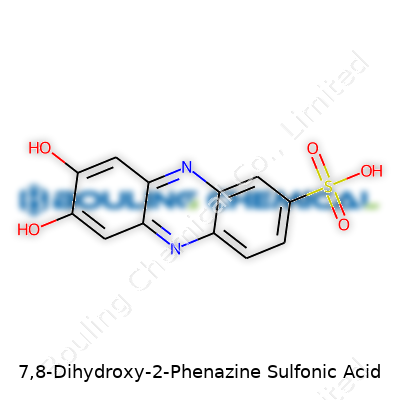
7,8-Dihydroxy-2-Phenazine Sulfonic Acid stands out for its deep color and strong electron exchange character, making it enticing to scientists working with both complex bioassays and redox-dependent sensors. It sports a phenazine backbone—a recognizably fused aromatic system—adorned with two hydroxyl groups at the 7 and 8 positions, and a sulfonic acid group at the 2 position. This molecule catches the eye for its intriguing mix of aqueous affinity and rigid aromaticity, two features not frequently combined. Its presence in research circles keeps growing, since the robust color it imparts aids in easy visualization and quantification across various scientific fields.
With a molar mass resting around 304.3 g/mol, the darkly colored crystals or powder produced by most labs testify to its potent conjugated structure. The sulfonic acid group at the two-position carries a negative charge at physiological pH, giving the molecule flexibility in water-based systems. The two hydroxyl substituents participate freely in hydrogen bonding, further enhancing water solubility and lending the molecule a touch of chemical versatility. Solubility experiments show good dispersal in water and aqueous buffers, less so in organic solvents like chloroform or hexane, and the acidity from the sulfonic group translates to a low pKa, boosting its performance as an acid catalyst or dye.
Reputable suppliers provide 7,8-Dihydroxy-2-Phenazine Sulfonic Acid under strict labeling, typically highlighting purity (often 98% or higher), moisture content, and identity confirmation through melting point and infrared spectroscopy. Chromatographic analysis, usually using HPLC, becomes standard to detect trace impurities and confirm batch consistency. Safety sheets point out the chemical’s robust stability under normal storage conditions. Clear labeling lowers risk, particularly in multidisciplinary labs where cross-contamination can throttle entire projects. Good labeling, to my mind, delivers confidence alongside quality, especially for those working on sensitive biological or analytical methods.
The most widely adopted route for preparing 7,8-Dihydroxy-2-Phenazine Sulfonic Acid leverages the sulfonation of the parent 7,8-dihydroxyphenazine. An excess of fuming sulfuric acid initiates direct electrophilic substitution at the two-position, favoring the most electron-rich site on the aromatic ring. After careful heating under controlled temperatures, the product gets neutralized with sodium carbonate, crystallized from aqueous ethanol, and subjected to repeated washing to eliminate trace mineral acids. Many in the lab world can recall the distinct odor of these reactions and the vibrant yellow-orange hues that mark each stage—a multi-sensory experience central to heterocyclic chemistry. Final purification often involves charcoal decolorization and vacuum drying, a process that teaches patience and a good nose for clean product.
The sulfonic acid and hydroxyl groups beg for further modification, and practitioners rarely miss that opportunity. The sulfonate tends to couple easily with amines; this helps graft the molecule onto solid supports for sensor applications. The hydroxyl groups open doors for alkylation, esterification, and oxidative coupling. These reactive centers underpin why every year, papers surface describing new conjugates for biosensors or next-generation antibiotics. Ullmann coupling enables extension of the aromatic system, and oxidative dehydrogenation, under mild conditions, tweaks redox capacity for energy storage work. Working hands-on, it becomes clear that every chemical group tacked onto this skeleton shifts its profile, forcing both excitement and respect for the unpredictable outcomes in multi-step syntheses.
Depending on supplier and research context, you might spot this compound listed as 7,8-Dihydroxy-2-phenazinemonosulfonic acid, Phenazine-2-sulfonic acid, or occasionally under code names like PHZSA-78. Generic codes crop up in regulatory filings, but most chemical databases stick to systematic names, ensuring anyone who works at a bench can cross-check molecular identity without confusion. These synonyms occasionally produce headaches, especially when translating between publications in different languages or from competing chemical catalogs, emphasizing the critical role of unambiguous nomenclature.
Anyone handling 7,8-Dihydroxy-2-Phenazine Sulfonic Acid in bulk soon learns that even the friendliest-seeming molecule—aromatic, colorful, not especially volatile—demands respect. Direct contact with skin or eyes triggers irritation, a reminder that aromatic sulfonic acids can punch above their apparent weight. Gloves, goggles, and fume hoods remain standard, especially during weighing, dissolving, or pouring. Environmental protection guidelines classify this chemical as a minimal hazard for aquatic systems, but good disposal practices remain non-negotiable. Most labs store the compound in tightly sealed amber bottles, protected from strong oxidizers and bases. Handling protocols, including spill management and basic first aid information, should feature prominently in every laboratory manual or chemical management plan.
Researchers turn to 7,8-Dihydroxy-2-Phenazine Sulfonic Acid for its powerful redox chemistry. In electron transfer assays, it’s a staple for monitoring enzyme function, notably in oxidative stress biology where precise detection of redox shifts changes everything for mechanistic insight. Analytical chemists use it as a key intermediate in colorimetric assays, where sensitivity down to micromolar levels matters. Its magnetic resonance makes it attractive for developing MRI contrast agents, while the same properties facilitate the design of next-generation biosensors for food safety and pharmaceuticals. I’ve seen colleagues put it through the wringer in studies of bacterial metabolism, banked on its structural resemblance to known antibiotics—sometimes with impressive leads for small-molecule hits against tough pathogens. The strong sulfonic acid group helps anchor it to hydrophilic surfaces, enabling everything from test strip assays to thin-film battery components.
R&D teams, both in academia and industry, treat 7,8-Dihydroxy-2-Phenazine Sulfonic Acid as a platform molecule that can adapt to new challenges as they emerge. Labs at the cutting edge of analytical diagnostics look for derivatives that fine-tune detection limits or boost biocompatibility. I once collaborated on a project that tested phenazine derivatives for solar cell stability, finding that the sulfonic acid groups stabilized the dyes against photobleaching, a chronic issue in earlier designs. Research continues into linking this molecule to antibodies and nucleic acids via the sulfonate, creating novel diagnostic reagents. Computer modeling teams analyze its conformational changes under various pH, searching for hidden possibilities in catalysis or drug development. The research push never really stops—across biochemistry, chemical engineering, and materials science, you find this molecule answering new questions every year.
Knowledge about the toxicity of 7,8-Dihydroxy-2-Phenazine Sulfonic Acid remains a work in progress, and this uncertainty shapes how both researchers and regulators approach its use. Initial studies show low acute toxicity in standard lab animals, but careful work underlines the importance of exposure time and route. The molecule’s aromatic nature has raised concerns about long-term mutagenicity and bioaccumulation, though the presence of strong water-solubilizing groups usually shortens its biological half-life. Metabolic breakdown studies, using liver microsomes, point to rapid conjugation and renal clearance, but comprehensive chronic toxicity assessments lag behind new applications. Practitioners follow the rule: better to use small-scale, well-controlled experiments, especially in applications that could touch food or drinking water. Funding for long-range mutagenicity and reproductive toxicity studies will determine how widely this molecule can be used outside of the lab.
The scope for 7,8-Dihydroxy-2-Phenazine Sulfonic Acid continues to widen as technology pursues new challenges in clean energy, next-gen bioassays, and anti-infective therapies. As needs grow for better electron transfer agents in bioelectronics and greener sensors, this molecule stands ready, with a strong legacy and a solid technical profile. Researchers see promise in creating targeted derivatives, using the molecule as a launchpad for antimicrobial, antiviral, and even anticancer agents. More collaboration among chemists, toxicologists, and regulatory experts will help the chemical world harness this compound safely. For tech startups, public research institutions, and pharmaceutical giants alike, practical solutions depend on more open data about synthesis, handling, and safety. Looking ahead, the push for both sustainability and innovation keeps 7,8-Dihydroxy-2-Phenazine Sulfonic Acid on the radar—compelling everyone involved to watch, adapt, and occasionally take bold chances in their experiments.
In the medical research world, some molecules hardly get a headline, but they quietly play crucial roles. 7,8-Dihydroxy-2-phenazine sulfonic acid, a synthetic compound with a mouthful of a name, has been grabbing more attention in recent years. My work in a university lab once exposed me to compounds just like this, and what stuck with me was how even the most obscure chemicals can change the direction of a project.
Researchers have studied this compound as an intermediate for redox indicators. In plain language, it helps signal when a chemical reaction has taken place by changing color or another property. In clinical labs, this behavior matters. Doctors, nurses, and lab techs rely on fast, accurate information about what's happening inside someone’s body. A color-change reaction that flags the growth of bacteria or the breakdown of blood sugars saves time and sharpens decision-making. According to the American Association for Clinical Chemistry, quick diagnostics have become even more important as patient loads climb and lab budgets shrink. Every shortcut that keeps care accurate buys time and, sometimes, saves lives.
Dyes and stains shape a lot of the chemical testing in facilities. The phenazine core of this compound is a workhorse in dye chemistry. Folks in textile manufacturing still rely on complex phenazine derivatives for certain colors that resist light and washing. Some of my friends in industry have described the headaches caused by finding alternatives when environmental guidelines change. This acid gives chemists another building block for water-soluble dyes, especially those with a sharp color that doesn’t fade under lab lighting. Researchers from Europe’s biggest dye suppliers report that small tweaks to molecular structure, including adding groups like sulfonic acid, can turn a standard dye into a specialty tool for cell staining, wastewater tracking, or anti-corrosive coatings in pipes.
Laboratories that screen for diseases like tuberculosis use stains derived from phenazines, since they bind to bacterial cell walls and expose infections under the microscope. Anyone who’s spent time behind a microscope knows the frustration of barely-there stains. Small adjustments in the chemical formula, like the addition of sulfonic acid groups, fix issues of poor contrast and weak signal.
Chemical companies design new 7,8-dihydroxy-2-phenazine sulfonic acid derivatives to serve greener manufacturing. In wastewater analysis, redox-active dyes flag contamination from metal-processing plants. I saw a small water utility in the Midwest switch to a system based on these dyes to stay ahead of EPA requirements. Once heavy metals build up, they don’t just threaten the rivers; they force expensive clean-ups. This compound helps provide timely warnings, letting engineers take action before problems get out of hand.
Making these chemicals raises its own questions about worker safety and environmental health. Responsible labs and factories follow strict handling protocols. The European Chemicals Agency and EPA both insist on tight tracking. There’s always space for better waste recycling, safer shipping practices, and transparent labeling. Partnerships between chemistry departments and public health experts help keep regulations strong and realistic.
Progress in science leans heavily on small discoveries. With 7,8-dihydroxy-2-phenazine sulfonic acid, the next big leap might come in the form of a faster hospital test or a cleaner industrial process. Each advance asks for honesty, safety, and accountability from researchers and industry. Folks who stay curious and careful are the ones who move things in the right direction.
Anyone who’s spent time working with sensitive organic chemicals will recognize the frustration that comes from a compromised batch. 7,8-Dihydroxy-2-Phenazine Sulfonic Acid falls squarely into that world where stability matters more than most realize. With two hydroxy groups and a sulfonic acid moiety, this molecule doesn’t just look vulnerable on paper—the risk of oxidation and the draw of ambient moisture often play out right in the lab or storage room. Basic chemistry tells us that air and sunlight love to make mischief with compounds like this one.
I learned the hard way in my research days just how fast a hygroscopic compound can clump or degrade, especially in a damp or warm storage closet. For this phenazine derivative, an airtight container is non-negotiable. Not just “sealed,” but truly airtight—think glass with Teflon-lined caps, or heavy-duty polypropylene with a solid gasket. Water in the air will turn the powder sticky, damage its purity, and set off a slow chain of reactions nobody wants.
Room temperature storage only works if that room stays dry and sits well below 25°C. Many chemists prefer the security of a cool, controlled fridge, ideally in the 2–8°C range. Take care to avoid self-defrosting refrigerators since they sometimes fluctuate in humidity and temperature. Desiccators backed with fresh silica or molecular sieve granules provide a second line of defense if you don’t have access to specialty climate control.
Photochemistry textbooks love to point out just how much fun certain aromatic compounds can have when the lights stay on. Keep 7,8-Dihydroxy-2-Phenazine Sulfonic Acid in amber glass bottles or, for extra caution, wrap containers in foil. Sunlight and even strong laboratory lights set off slow oxidation and possible color changes—none of which help research reproducibility or product safety.
Fresh air is no friend in this context. Each opening of the bottle risks letting oxygen and water vapor slip in. Always portion out what you’ll use instead of daily opening the main stock. If you work in a setting where glove boxes are a norm, use them to dispense or weigh out the chemical. It sounds picky, but small changes in oxygen exposure matter over months of storage.
Not all batch failures come from the obvious accidents. Forgetting when a bottle was opened or failing to mark the storage date ruins many good chemicals. Always include the date received and date opened right on the label. Short-term stock—opened or frequently handled—should get used up within six months. Unopened, properly sealed bottles, stored in strictly controlled environments, give reliable results for one to two years, but always check the manufacturer’s recommendation.
7,8-Dihydroxy-2-Phenazine Sulfonic Acid’s sulfonic acid group boosts water solubility, raising questions about environmental release. Nobody wants to see specialty chemicals entering the wastewater stream. Follow your facility’s guidelines for hazardous waste—neutralize if required, never flush down regular sinks, and always document your disposal.
Lab workers and chemical suppliers share a responsibility to protect both the quality of their reagents and the safety of the environment. Simple practices—airtight storage, dry and cool environments, regular checks on expiration, and sensitive waste handling—are the backbone of responsible handling of chemicals like this one. Paying attention saves money, time, and, most importantly, keeps experiments honest.
Ask anyone in a lab, a factory, or even an artist’s studio about why purity and grade stand out so much. The answer comes from lived experience. Picture working on a reaction for a new material. Everything’s set up, calculations checked, then a tiny impurity in your solvent flips the result. You only notice after wasting hours, sometimes days, backtracking and troubleshooting. That one misstep traces back to the chemical’s grade.
A product grade isn’t just a tag. It signals how much trust we can put into what’s inside the bottle. In practice, it decides whether a solution is safe for medicine, decent for washing lab glass, or best kept far from food or skin. Every field sees it this way. Analytical chemists expect “reagent-grade” to mean interferences stay out of their measurements. Manufacturers demand “pharma-grade” to avoid guesswork with drug safety. Artists and hobbyists pick “technical grade” for cost, knowing there’s a trade-off.
Here’s a story from working with undergrads in a teaching lab. Someone wanted to use industrial sulfuric acid for a battery prototype, thinking it’d save money. What a surprise to learn that iron traces in the cheaper product stopped the battery from holding a charge. That’s more than just a financial setback. Impurities don’t always announce themselves with colored streaks or odd smells. Sometimes, their effects run silent, like toxins or explosive byproducts. Improper chemicals in foods or drugs spark product recalls, emergencies, and lawsuits — none of which any company wants to face.
“99% pure” looks impressive, but the last 1% can be trouble. Let’s say a chemical is listed at “ACS Reagent grade, 99.5%.” For most experiments, that 0.5% sounds tiny. If you’re making a semiconductor, though, even a few parts per million can shift the physics and waste an entire production run. For pharmaceuticals, a single contaminant above the threshold means starting over. Knowing a product’s certificate of analysis and supplier track record helps avoid nasty surprises.
Every time cost pressures push for a lower grade, real-world consequences come knocking. In the long run, strict documentation wins. Suppliers who publish independent test results, disclose trace impurity levels, and keep detailed batch records deserve attention. Rather than hoping for the best, smart labs request the “certificate of analysis” for each batch. Cross-checking analytical data with the supplier builds trust while protecting people and budgets.
For anyone mixing, measuring, or formulating, getting in the habit of reading labels, certificates, and supplier histories isn’t just best practice; it’s basic survival. The cost savings from using lower-grade chemicals often vanish as failed batches, safety incidents, or regulatory headaches pile up. Experience teaches: purity and grade aren’t negotiable. They shape everything from a simple classroom demo to multi-billion-dollar manufacturing. Keeping an eye open for genuine certificates, quality testing, and supplier transparency leads to better outcomes, fewer surprises, and safer work for everyone involved.
Anyone who’s stepped into a chemistry lab or a specialty manufacturing plant knows how easily things can go wrong with complicated chemicals. I remember the stinging smell of unknown powders on my glove after a long shift—how a clumsy moment could burn or blister if I didn’t pay attention. Handling 7,8-Dihydroxy-2-Phenazine Sulfonic Acid, with its tongue-twister of a name, is about keeping routine, supplies, and habits tight. Mistakes around strong chemical agents cost dearly, whether it’s a worker’s skin, their lungs, or the safe track record of a workplace.
With substances like this, gloves aren’t just for show. Nitrile or butyl rubber gloves block most organic agents, unlike the cheap latex ones you find at the corner store. Full coverage lab coats, safety glasses—or better yet, splash-proof goggles—and sturdy shoes with closed toes become your daily uniform, not optional extras. I learned to double-check that sleeves are tight and nothing from your forearms gets exposed. Sometimes we joked about looking like beekeepers, but there’s nothing funny in chemical burns.
When I worked with strong acids, proper ventilation kept vapors from hanging heavy in the air. Localized fume hoods pull away airborne material before anyone breathes it in. If you work someplace without this, bring up the need for it in team safety meetings. Every worker deserves clean air, and basic fans just move the problem around.
Tossing containers on a regular shelf turns your storeroom into a hazard zone. Separate 7,8-Dihydroxy-2-Phenazine Sulfonic Acid from any strong bases or oxidizers. I’ve seen plain old cardboard melt or weaken after chemical exposure, so stick to sealed glass or certain plastics designed for aggressive chemicals. Label everything with bold, waterproof ink—more than once, faded notes on containers led to panicked scrambles.
Spills happen, but how you deal with them matters. Absorbent pads and neutralizing agents should sit within arm’s reach of any bench or workstation. It’s tempting to rush, but I learned to breathe, close off the area, and get out the spill kit before trying to touch anything. Protecting yourself and your team always comes before cleaning up for the clock or the boss.
Scientific literature points to possible skin and respiratory risks. Chronic contact with phenazine compounds has sometimes led to allergic reactions and inflammation. I trust published research and experience shared by colleagues. Each safety manual tells some of the story, but real-world habits and the lessons passed down from previous staff fill in the gaps. If questions come up—“Will this chemical dust get through these gloves?”—call the supplier. It’s never a waste of time.
Having firsthand experience in both classroom and hands-on settings, I’ve seen how dry safety talks leave folks half-listening. Emergency drills that put gloves on hands and eyewash stations within reach stick better in memory. Clarify the emergency plan: know where to run, who to call, and how to pull someone out of a dangerous spot. One properly stocked eyewash station has saved a coworker’s sight. Keeping the right posters up and safety data sheets at the ready gives everyone a fighting chance if something goes wrong.
I’ve watched new staff learn to respect 7,8-Dihydroxy-2-Phenazine Sulfonic Acid, not fear it. Safe handling comes from small, steady habits: changes of gloves after spills, keeping lab benches clear, swapping tips between shifts. Every job worth doing brings risk. Facing it together, learning from mistakes, and pressing for good resources—these actions protect everyone and let skilled teams focus on real work, not damage control.
Chemistry shapes a lot of everyday life, even when it hides behind complicated names like 7,8-Dihydroxy-2-Phenazine Sulfonic Acid. Some see a mouthful, but those who work in medicine, environmental science, and material synthesis see a gateway to all sorts of innovations. Understanding its makeup means unlocking how it acts in reactions, how it interacts with other compounds, whether it’s safe, and what jobs it can do. Anyone working with similar molecules—either in a research lab or in industry—finds these details practical.
This compound’s chemical formula often shows up as C12H8N2O5S. Easy enough to write, but behind those letters and numbers sits a detailed map of atoms joined up in a precise way. The base, phenazine, forms a fused ring system, giving it stability and a platform for further modification. Add two hydroxy groups at the 7 and 8 positions—they change the electron distribution in the aromatic ring, which can tweak reactivity. The sulfonic acid group at the 2-position increases water solubility, makes the compound less volatile, and offers a spot for making industrial dyes or certain pharmaceuticals.
You do not need to be a full-time chemist to see why knowing the exact formula makes a difference. Analytical chemists rely on it when running mass spectrometry or nuclear magnetic resonance scans, pharmaceutical developers need it to file regulatory documents, and environmental scientists track molecules like this to check their breakdown and persistence in water.
The molecular weight—308.27 g/mol for C12H8N2O5S—looks like another stat, but it’s much more. For chemists, this number guides every reaction calculation. You want to make a solution? You reach for this number to know how many grams to weigh out for a desired molarity. Lab teachers walk students through using it over and over until it becomes second nature. In pharmaceutical development, dosing hinges on having this exact value right down to the decimal, since it determines the strength and efficacy per pill or vial.
Scientists also use molecular weight to compare compounds when they try to make reactions more efficient or when finding alternatives for safer environmental impacts. Smaller molecules sometimes move through membranes more easily or break down faster, while heavier ones might linger and build up in places they shouldn’t.
A rigorous approach to fact-checking pays off. Uncertainties in chemical formula or weight can mean wasted batches in manufacturing, failed medical trials, or inaccurate toxicity reports. Mistakes in the numbers might cause a batch of medication to work too weakly or become toxic. In industrial chemistry, a tiny error multiplies when production scales up, resulting in thousands of kilograms of off-spec material.
Double-checking numbers against trusted databases—like PubChem, ChemSpider, or peer-reviewed chemical catalogs—prevents errors. Following good documentation and sample labeling matters just as much on the academic bench as in commercial plants.
Chemicals like 7,8-Dihydroxy-2-Phenazine Sulfonic Acid attract attention in dye research, water treatment studies, or drug synthesis. Each field wants clear and confirmed data. Keeping accuracy central fuels discovery, keeps users safe, creates trust, and reduces risk of regulatory headaches later on.
| Names | |
| Preferred IUPAC name | 8-hydroxy-7-oxo-7,8-dihydrophenazine-2-sulfonic acid |
| Other names |
8-Hydroxyphenazine-7-sulfonic acid NSC 113927 Phenazin-2-sulfonic acid, 7,8-dihydroxy- 7,8-Dihydroxy-2-phenazinesulfonic acid |
| Pronunciation | /ˈsɛvən eɪt daɪˈhaɪdrɒksi tuː fɪˈnæziːn sʌlˈfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 5729-52-8 |
| 3D model (JSmol) | `3D_FF:C1=CC2=C(C(=C1O)O)NC3=CC=CC=C3N2S(=O)(=O)O` |
| Beilstein Reference | 285758 |
| ChEBI | CHEBI:134413 |
| ChEMBL | CHEMBL3912331 |
| ChemSpider | 21985617 |
| DrugBank | DB08625 |
| ECHA InfoCard | 100.036.368 |
| EC Number | 4.2.2.8 |
| Gmelin Reference | 85586 |
| KEGG | C16516 |
| MeSH | D015438 |
| PubChem CID | 160658 |
| RTECS number | SS9625000 |
| UNII | SF8T2BZ2KE |
| UN number | 2811 |
| CompTox Dashboard (EPA) | DTXSID2061587 |
| Properties | |
| Chemical formula | C12H8N2O5S |
| Molar mass | 350.33 g/mol |
| Appearance | brown powder |
| Odor | Odorless |
| Density | 1.68 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -0.44 |
| Vapor pressure | 3.93E-11 mmHg at 25°C |
| Acidity (pKa) | 6.5 |
| Basicity (pKb) | 7.76 |
| Refractive index (nD) | 1.754 |
| Dipole moment | 5.12 Debye |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH⦵298) | -9.83 kJ/mol |
| Pharmacology | |
| ATC code | D05AX01 |
| Hazards | |
| Main hazards | Causes serious eye damage. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 1, Instability: 0, Special: - |
| NIOSH | Not listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5 mg |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Phenazine Phenazine methosulfate Neutral red |