The story of 5-Thiazolylmethanol goes back to the rush of 20th-century heterocyclic chemistry. Scientists recognized thiazole rings as foundational blocks for antibiotics, dyes, and countless organic molecules that changed both medicine and industry. Early synthetic methods surfaced as researchers needed to explore sulfur and nitrogen chemistry. Over years, improvements in handling thioureas and α-haloketones opened doors for more reliable thiazole production—including various substituted thiazolylmethanols. Labs in Europe, the US, and Japan often competed to build libraries of functionalized heterocycles, promoting the use of 5-Thiazolylmethanol in targeted drug and agrochemical research. While large companies sought intellectual property, small synthetic groups honed their craft through ingenuity and improved safety protocols. By the early 2000s, automation and computation had streamlined its creation, making this compound much more accessible to academic and commercial chemists alike.
5-Thiazolylmethanol offers a distinct blend of reactivity and stability. As a colorless to pale yellow solid or viscous liquid, depending on purity and storage, it’s favored for introducing both thiazole and primary alcohol groups to target molecules. Its thiazole ring attracts interest for bioisosterism, mimicking other aromatic structures yet providing different electronic effects. These features give medicinal chemists genuine utility: modifying activity, tuning solubility, and impacting metabolic fate. This molecule doesn’t attract attention only for complexity—it succeeds in practice, especially when projects demand incremental substitution or desire a bioactive core scaffold.
5-Thiazolylmethanol typically appears as a low-melting solid under ambient conditions. The molecular formula is C4H5NOS, with a molar mass close to 115.16 g/mol. Its melting point generally spans from 45°C to just under 60°C, while it begins to decompose above 180°C. Water solubility stands out—it mixes sparingly in cold water but much better in alcohols or polar aprotic solvents such as DMSO or DMF. The structure gives 5-Thiazolylmethanol a slightly aromatic odor and puts it on the mild end of volatility. With a free hydroxymethyl group bonded directly to the ring, the molecule welcomes classical alcohol modifications, and the ring sulfur lends distinctive spectral signatures in NMR and IR. It withstands routine handling but does not fare well against strong oxidizers, acids, or conditions facilitating ring cleavage.
Specifications for 5-Thiazolylmethanol focus on purity, trace metal content, and defined limits for multi-compound impurities. Purity above 98% is standard for research labs; process chemistry may tighten this threshold for pharmaceutical intermediates. Analytical certificates identify method (often HPLC, GC, or NMR) and storage must maintain temperatures below 25°C, away from direct sunlight. The packaging requires chemical-resistant materials—amber glass vials for small quantities or HDPE bottles for production lots. Safety markings follow GHS protocols: hazard pictograms, risk phrases, and instructions for safe use. Unique identifiers like CAS number 18800-36-5 and batch/lot codes ensure traceability. Producers sometimes add tamper-evidence or QR labels for digital access to technical documentation, a helpful step in maintaining Good Manufacturing Practice chains.
Synthetic approaches to 5-Thiazolylmethanol use varied starting points, though most rely on cyclization between α-haloketones and thiourea or its derivatives. One traditional route takes 2-chloroacetylaldehyde, shifts its reactivity with thiourea, and closes the ring while generating the alcohol functionality. Another involves 5-methylthiazole first, followed by targeted oxidation and hydroxymethylation. Careful temperature control and staged purification prevent over-oxidation or ring degradation. Some modern labs harness microwave-assisted synthesis to shrink reaction times from hours to minutes. Purification usually combines liquid-liquid extraction with column chromatography or fractional crystallization. Every route carries trade-offs in yield, solvent load, and scalability—factors weighed closely in commercial settings versus university work. Waste management and worker safety now sit central to every process, as green chemistry principles push manufacturers to opt for lower-toxicity routes and reusable catalysts.
Chemists rarely leave 5-Thiazolylmethanol untouched. The primary alcohol group gives direct access to esters, ethers, or oxidized products like aldehydes and acids. Phosphorylation is common in bioorganic work, prepping the molecule for nucleoside analog synthesis. Carbamate and sulfonate formation offer further functionalization. The thiazole ring, reactive at both the 2- and 4-positions, encourages substitution—especially in metal-catalyzed cross-coupling reactions (Suzuki, Heck, etc.). Electrophilic aromatic substitution selectively targets the carbon, opening paths to arylated or halogenated derivatives important for libraries. Direct lithiation sometimes threatens ring stability, so milder conditions often win out, using milder Lewis acids or photoredox approaches. These combinations foster new molecular diversity, supporting thousands of patent filings each year for pharmaceuticals, herbicides, and advanced materials.
5-Thiazolylmethanol goes by several names across suppliers, literature, and patent filings. Its IUPAC designation lands as (Thiazol-5-yl)methanol, though some catalogs list it as 5-(Hydroxymethyl)thiazole. Trade sources might shorten this to Thiazole 5-methanol or keep the numeric priority intact. Synonyms sometimes reflect salt or hydrate forms, depending on isolation method or downstream formulation. While its CAS number (18800-36-5) cuts through ambiguity, regional variations in language or supplier conventions sometimes blur things—especially in translations from non-English protocols. Accurate identification in an order, customs form, or research paper demands attention to all possible aliases.
Working safely with 5-Thiazolylmethanol means understanding its dual nature: low-to-moderate acute toxicity, minimal volatility, and the potential for skin and eye irritation. Researchers rely on gloves (nitrile or neoprene), splash goggles, and ventilation instead of fume hoods for most manipulations. Spills absorb easily into inert pads; waste drops in solvent disposal streams. Labels must warn against ingestion, inhalation, and contact with oxidizers. Companies codify operating procedures in safety data sheets, noting incompatibility—strong acids, oxidizing agents, and bases. Despite its modest risk profile, long-term toxicological data stays sparse, so conservative handling fits my own experience. Lab audits stress inventory control, spill drills, and annual review of PPE. For larger batches or pilot plants, closed systems and online detectors have become non-negotiable. Regulatory agencies sometimes tighten storage and use controls based on new research, especially in workplaces with vulnerable workers or young trainees.
Drug discovery makes wide use of 5-Thiazolylmethanol. It provides a core ring for anti-infective candidates, kinase inhibitors, and methyltransferase blockers. Agrochemical producers see value in its ring system for developing fungicides and growth regulators by tailoring lipophilicity and metabolic breakdown. Material scientists turn to the thiazole ring for modified resins, sensors, and as an intermediate for specialty dyes. Biologists convert it into labeled probes for enzyme studies—attaching fluorophores or metal-binding groups. In protein science, derivatized thiazoles mimic natural cofactors. Small biotech companies prefer it for rapid analog synthesis due to reliability and straightforward modification. Organic electronic research sometimes spins out new applications, relying on the aromaticity and reactivity of the thiazole system to alter conductive properties or photoemission. These fields have taught me firsthand to value compounds that meet both creative and reliability demands, and 5-Thiazolylmethanol proves itself again and again under pressure.
Current research circles around a deeper understanding of thiazole bioactivity—how modifications on the ring and side chain alter pharmacokinetics and toxicity. Automated screening makes quick work of small-molecule libraries, including a growing set of thiazolylmethanol analogs. Computational chemists spend hours simulating electronic distributions and predicting metabolic pathways, trimming the work needed for trial-and-error synthesis. In synthetic biology, researchers now tweak microorganism genomes to biosynthesize thiazole derivatives by fermentation, challenging traditional batch chemistry. Multinational companies sponsor collaborative studies to clarify environmental breakdown, human exposure, and long-term persistence. The race to patent space for new kinase inhibitors or herbicide actives keeps commercial R&D teams pivoting fast. In my own experience, cross-functional teamwork finds the greatest productivity—pairing synthetic insight with biological interpretation, and letting analytic experts spot the best candidates for scale-up.
Studies on 5-Thiazolylmethanol’s toxicity remain limited but insightful. Standard acute toxicity tests in rodents suggest modest oral and dermal hazards—not on par with strong toxics, but worth prudent handling. Detailed mutagenicity screens turn up few alarms, though no insurer accepts this as a clean bill of health. One concern: its metabolic byproducts can stress liver enzymes in some animal models, drawing comparisons to structurally related heterocycles. There’s little evidence for persistent environmental toxicity; soil bacteria tend to break down thiazole-containing residues over weeks. Occupational exposure at high concentrations—airborne dusts or solvent splashes—hasn’t led to systemic poisoning reports, though chronic inhalation draws caution. The absence of clear human data encourages researchers and companies alike to keep safety limits conservative and update protocols as science fills gaps. Peer-reviewed journals keep watch for case reports or industrial mishaps—none significant yet, but science demands vigilance.
5-Thiazolylmethanol promises to remain a staple as organic chemistry embraces more sustainable and modular methods. Expansion into green chemistry—reducing solvent use, recovering reagents, and exploring biocatalysis—keeps the compound relevant. Its role in medicinal chemistry stands likely to grow, especially as researchers need more unusual scaffolds to address resistance and off-target effects. Synthetic biologists and chemical engineers explore cost-effective fermentative routes, aiming for “sugar-to-thiazole” flowsheets that could replace traditional, resource-heavy syntheses. As regulatory pressure mounts for better toxicity profiles, companies search for analogs with faster environmental breakdown while keeping desired activity. In digital healthcare, researchers feed structural data on 5-Thiazolylmethanol and siblings into machine learning models seeking new targets. Diversity in application, safety in process, and ongoing innovation promise a strong future for this humble yet powerful molecule. My own experience working with derivative synthesis teams shows little sign of complacency—this field rewards curiosity, safety, and collaborative drive, making the next chapter of 5-Thiazolylmethanol’s story one to watch.
5-Thiazolylmethanol does not sound like something you come across in a grocery store aisle. For folks working away in chemistry labs or chasing breakthroughs in pharmaceuticals, this compound earns a place on their radar. Its thiazole ring, a key structure tucked into its name, pops up across many research projects because of how this small molecule packs a surprising punch.
Many people never hear about 5-Thiazolylmethanol in school, yet it serves as a foundation in some serious research. Chemists work with this compound to create new synthetic molecules. These new creations might become tomorrow's treatments for infections, pain, or even cancer. The thiazole ring—five members strong, including a sulfur and a nitrogen atom—plays a starring role in many well-known drugs, like the beta-lactam antibiotics we lean on to fight off bacterial infections. Making small tweaks to this base structure, including changes built from 5-Thiazolylmethanol, gives chemists a way to search for better and safer medicines.
Researchers constantly hunt for molecules with specific properties, like the ability to disrupt bacterial growth or help cells talk to each other in new ways. 5-Thiazolylmethanol offers a handy starting point for building these molecules. Medicinal chemists often use it to design new antibiotic possibilities, antiviral candidates, and even potential drugs to target inflammation or tumors. In the search for new antibiotics—a real and growing need as drug resistance outpaces discoveries—scientists test hundreds of tweaks to basic structures. Without compounds like 5-Thiazolylmethanol on their shelves, these searches slow down to a crawl.
It does not stop at pills and injections. 5-Thiazolylmethanol can become part of lab tools used by biologists to understand how enzymes work. Some teams create dyes or sensor molecules with this scaffold to help track what is happening in living cells. Every improved test in a basic science lab, every new marker that helps spot disease early, has someone behind the scenes working with building blocks like 5-Thiazolylmethanol.
We all depend on the progress happening behind closed lab doors. Watching my friends try to solve the puzzle of antibiotic resistance or better cancer drugs, I have seen how tiny building blocks like this one matter. A breakthrough in the lab can ripple out and touch lives for decades. Sometimes, a project takes off only because a researcher found the right version of a molecule like 5-Thiazolylmethanol on the chemical supply shelf. Every compound in a chemist's toolbox has the potential to shift how we treat disease or detect health issues, especially as problems like antibiotic resistance press harder on our health system.
The push for new and safer drugs keeps this small molecule in circulation. Open data, shared chemical libraries, and partnerships across universities and industries can help more researchers tap into the full power of building blocks like 5-Thiazolylmethanol. Cheaper, safer, and easier access to these tools will let bright ideas move from theory to practice, turning today’s chemical experiments into tomorrow's health breakthroughs.
From a background in pharmaceutical research, I learned early on how much impact a small change in molecular structure can make. 5-Thiazolylmethanol stands out as one of those molecules that grabs attention for its core component, the thiazole ring. This five-membered ring contains both sulfur and nitrogen, places the backbone for a lot of biological compounds, and it’s not just a rare feature in nature—life relies on variations of this ring for things like vitamin B1 (thiamine).
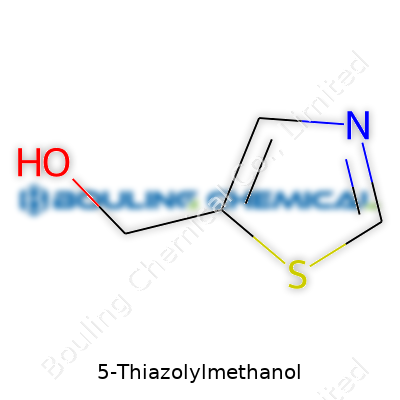
The name almost spells out the build: 5-Thiazolylmethanol. “Thiazolyl” points to the thiazole ring. The “5” means something attaches at the fifth spot on that ring. Adding “methanol” means there’s a –CH2OH (hydroxymethyl) group. Chemically, the structure matches C4H5NOS for the base thiazole, with the CH2OH group lengthening it to C5H7NOS.
Drawing it out on paper always helps—picture a pentagon representing the ring. Sulfur occupies position 1, nitrogen sits at position 3. You find the –CH2OH group attached directly to position 5, meaning this spot is where chemists start tailoring the molecule for new uses. I’ve spent months in labs sketching out similar structures by hand, measuring bond angles, and the difference that one group makes on the end can change solubility or reactivity drastically.
Getting specific about the thiazole ring is not just chemistry trivia. Medical researchers often explore thiazole derivatives thanks to their recurring presence in antibiotics, antifungal agents, and even antitumor candidates. People with an interest in drug development often start by modifying groups just like the methanol branch at position 5. They do this to improve how drugs move through the body, avoiding breakdown too quickly or hanging around just the right amount of time. The hydroxymethyl group often adds a boost to water solubility, making the compound a little easier to formulate.
There’s a long track record showing why these building blocks matter. Thiazole itself anchors molecules fundamental to life, like the vitamin B1 molecule that’s central to energy metabolism in humans and animals. A tweak at position 5 can open up a pathway to drugs that catch the attention of major pharmaceutical companies, especially with growing concern about antibiotic resistance. I’ve seen collaboration between chemists and biologists lead to new uses by focusing on analogs of simple structures like 5-Thiazolylmethanol.
Lab chemists often look for ways to make 5-Thiazolylmethanol straightforwardly, choosing synthetic paths that avoid hard-to-handle intermediates. One method I’ve used relies on forming the thiazole first, then adding the methanol group through targeted reactions. This route supports scalability, which turns out vital when shifting from gram-scale synthesis to industrial production.
For researchers, documenting each property—the melting point, reactivity, solubility—keeps projects on course. Reliable data means better reproducibility and fewer surprises down the road. The structure, with its thiazole core and side chain, offers a familiar starting point with room to customize for needs in everything from medicinal chemistry to special-purpose dyes and sensors.
This core molecule reconnects us to both fundamental science and fresh innovations, driven by small molecular tweaks and the pursuit of real-world impact.
Anyone who’s ever spent time in a research lab knows that storing chemicals isn’t just about sticking bottles on a shelf. It’s about respect for the material and staying safe. 5-Thiazolylmethanol, a compound with a thiazole ring and a hydroxymethyl group, isn’t the sort you treat casually in you storage plans. A bit of carelessness turns a useful reagent into a safety hazard or a lost investment.
I recall the frustration of discovering spoiled reagents after simple neglect. With 5-Thiazolylmethanol, the safety data sheets point right at cool, dry conditions—15°C to 25°C is a comfortable range. Anything much warmer means decomposition might creep up on you. There isn’t any call for refrigeration unless the label tells you, but a spot kept away from the radiator or fluctuating windows makes sense. This isn’t stubbornness; exposure to heat can cause unstable compounds to break down or even turn a low-risk chemical into a potential irritant.
You never really appreciate how big a deal moisture is until a fine white powder comes out caked, yellow, or plainly ruined. 5-Thiazolylmethanol doesn’t play well with humidity. A desiccator or air-tight bottle does the trick, blocking out the kind of dampness that runs wild in basements and careless storerooms. Researchers who check their containers once in a while spot caking early, which tells you water’s creeping in. Tackling this before any change in physical appearance sets in prevents both waste and confusion on your next experiment.
Some chemicals announce their sensitivity by fading in sunlight, and others get unstable in UV’s company. It's the same game for many thiazole derivatives, so amber bottles or storage out of the light keeps things as they should be. I’ve heard about folks who cover shelves in heavy cloths, but most times, simply using a dark cabinet or dedicated storage drawer works fine. The big cost of ignoring this isn’t just wasted money; degraded chemicals cause irreproducible results and sometimes hazardous by-products.
Not every bottle agrees with its roommate. 5-Thiazolylmethanol shouldn’t share a shelf with strong acids, bases, or oxidizing agents. Corrosives turn a clean bottle into a crusty mess, while powerful oxidizers have caused enough small lab fires for the rule to make sense. My habit is to group chemicals by hazard class and keep flammables, oxidizers, and bases in marked, separated storage. It isn’t always about dramatic reactions—sometimes, it’s about tiny leaks leading to slow degradation or unwanted by-products.
One clear, permanent label with the date of receipt, date opened, and source beats any memory trick. Small labs sometimes skip this, thinking they’ll remember, but after a month or two, even the best recall gets fuzzy. Most labs with good track records show thorough paperwork and inventory systems, not just to please inspectors, but because it protects everyone in the building from mystery bottles and unknown hazards.
I learned early on from a mentor never to open two similar-looking bottles at the same time, especially with compounds like 5-Thiazolylmethanol. Glove changes, one label at a time—these slow practices stop costly mix-ups and cross-contamination. No one wants to repeat an experiment just because their chemicals picked up a trace contaminant from next door. Checking expiry dates and levels every quarter means you never get caught off guard by an empty or outdated bottle. It’s about keeping the work honest and the lab safe.
Fresh, dry air, no direct sun, far from chemical rivals, and sharp labels—these make for a good storage plan. Spend a few minutes upfront and problems rarely follow. Good habits in chemical storage, learned from hard experience, shield valuable reagents and, even more importantly, keep a safe lab environment for everyone involved.
5-Thiazolylmethanol stands as a lesser-known chemical outside of research labs, but folks working in pharmaceutical development or chemical manufacturing might spot its name on an order sheet. The structure sounds fancy, though it boils down to a thiazole ring attached to a methanol group. This group of chemicals can pop up as building blocks in medicine or advanced materials. The point is, 5-Thiazolylmethanol serves as a tool, not a household product.
Hazardous chemicals come in all shapes and sizes, and 5-Thiazolylmethanol draws some attention for its thiazole core. Thiazoles, as a class, show up in lots of bioactive molecules. In my years in the chem lab, I learned to respect these compounds. Even subtle changes in structure can mean a world of difference in how safe—or unsafe—a substance becomes.
According to Safety Data Sheets from chemical suppliers, 5-Thiazolylmethanol usually gets flagged for eye, skin, and respiratory irritation. Most of the caution comes from its chemical relatives. Swallowing, inhaling, or letting these compounds soak into your skin is never wise. That warning doesn’t single this one out as worse than other lab reagents, but nobody enjoys a coughing fit or a rash from slopping liquids aroud.
Some folks worry any thiazole means high toxicity. That’s not the way chemistry works. Animal studies on 5-Thiazolylmethanol itself don’t show up much in public research. Looking at similar compounds with thiazole rings, most aren’t deadly at low doses, but they all earn labels like “handle with gloves and goggles.” In a lab, a little respect goes a long way. Touching or breathing chemical powders in an open bench can lead to nasty symptoms, from headaches to nausea or worse. The methanol side chain doesn’t make things safer, either, since methanol’s own toxicity record is well known.
EPA and OSHA haven’t made special rules just for 5-Thiazolylmethanol, at least not in the US. But their guidance tends to sweep up less common chemicals with broad safety advice. If the MSDS says “likely harmful if swallowed,” it’s hardly a green light for careless dumping or casual use.
Lab stories make the news mostly when people break the rules. I remember a colleague who shrugged off gloves and got a chemical burn. He didn’t think the stuff was serious until his hand started itching, then swelling. Mishaps don’t usually come from monster toxins—just common substances handled thoughtlessly. 5-Thiazolylmethanol joins that list. Regular lab protocols—ventilation, gloves, goggles, proper storage—cut risk to nearly zero.
Disposal brings its own issues. Flushing leftovers or soaking rags in a janitorial sink threatens water quality down the line. Thiazoles can degrade, but sometimes the breakdown products are even more worrisome for wildlife. Chemical suppliers and universities stress using authorized hazardous waste services. Nobody wants to see fish turn up dead because of sloppy habits.
5-Thiazolylmethanol tells a story about respect for chemicals, not runaway panic. Give any unfamiliar chemical proper attention. Read the safety sheets, talk with more experienced hands, and keep emergency plans within reach. Engineers look for safer alternatives, too. Substituting less toxic starting materials or putting in containment equipment makes a huge difference. Most risks shrink fast with a little know-how and a focus on preparation.
Chemists spend long days making sure every bottle that leaves a lab actually delivers what the label promises. Purity stands as a kind of trust between producer and scientist. For 5-Thiazolylmethanol, most inquiries come from researchers and manufacturers who need straight answers about what’s in their hands. In almost every reputable catalog, this compound typically appears with purity values above 98%. Nobody wants to lose hours in the lab because of a contaminated batch.
Lab work rarely gives second chances. Trace contaminants muddies results, triggers false alarms in experiments, and, in the drug industry, can even derail an entire project. Purity levels reported for 5-Thiazolylmethanol don’t just cater to chemists’ obsessions with numbers. Precise measurements reflect months—sometimes years—of process development in chemical manufacturing. Each decimal point signals a direct reflection of performance in every downstream application: synthesis, biological assays, or pharmaceutical research.
During my years in student research, I learned firsthand that low-grade chemicals derail weeks of effort. No one explains how disheartening it feels to run a spectrum only to spot background peaks from solvents or byproducts. The gold-standard value above 98% for this compound means fewer headaches downstream.
Quality doesn’t just appear overnight. Every lot of 5-Thiazolylmethanol passes through a set of checkpoints: melting point checks, high-performance liquid chromatography (HPLC), nuclear magnetic resonance (NMR), elemental analysis, and moisture content measurements. These methods verify whole identity, not just numbers on a spec sheet. Seasoned manufacturers track every variable in their synthesis—temperature, solvents, cleaning cycles. This vigilance pays off. A small change in process often changes product specs, and that’s where regular testing keeps customers confident.
What surprised me most early in my career was just how much the small steps matter. Tiny slip-ups in glassware cleaning or solvent reuse become major problems. Companies that deliver high-purity 5-Thiazolylmethanol run dedicated equipment and develop detailed protocols for purification and packaging. Glass bottles sealed tightly, tamper-proof seals, and inert gas blanketing all help keep things stable on the shelf and in transit.
A batch of 5-Thiazolylmethanol leaves the factory packed with paperwork, and experienced buyers know how much this matters. A decent supplier makes sure each vial ships with a clear certificate of analysis (COA)—not a generic printout, but a record of batch number, lot-specific purity, analytical method, and date. That’s where traceability steps in: any issues, and someone can follow the trail back through every step in manufacturing, shipping, and handling.
Analytical transparency matters. Some labs insist on HPLC chromatograms sent along with the product; others foot the bill for independent verification. That’s not paranoia—it’s hard-earned experience from scientists who have seen contaminated or mislabeled chemicals ruin entire thesis projects.
With so many labs relying on exact results, open communication between chemists and suppliers keeps standards high. It isn’t just about hitting a number above 98%, but about shared confidence, ongoing communication, and the hard work of routine testing. Good companies aren’t afraid to discuss process changes, share analytical details, or provide follow-up support.
During collaborations across different labs, I always paid close attention to how suppliers addressed questions. Openness and detailed answers show a real commitment to quality, far above just listing a number on the website. For 5-Thiazolylmethanol, as with any lab reagent, the story doesn’t end at the COA—it continues in every experiment that works as planned.
| Names | |
| Preferred IUPAC name | [(Thiazol-5-yl)methanol] |
| Other names |
5-Thiazolemethanol 5-Thiazylmethanol Thiazol-5-ylmethanol |
| Pronunciation | /faɪ-θaɪˈaːzɒl-ɪlˈmɛθ.ə.nɒl/ |
| Identifiers | |
| CAS Number | [504-35-8] |
| 3D model (JSmol) | `4d8J2fekbc4JgFF2wE2wFESc6Md6xwfBt1wFdFIsomAJmWZ44JqGnGEaGvQ63yGR2+dSyEKyYqAqJ2PbYqAUU7Rexryxld2GLCljytt2C2PxrCOr/gyhk9TDZRLX9eT94Rij6L9K89pSf9KMy70wIg5y9eOWn` |
| Beilstein Reference | 1305952 |
| ChEBI | CHEBI:34487 |
| ChEMBL | CHEMBL116647 |
| ChemSpider | 126926 |
| DrugBank | DB08369 |
| ECHA InfoCard | ECHA InfoCard: 100.045.349 |
| EC Number | EC 200-711-0 |
| Gmelin Reference | 81874 |
| KEGG | C06012 |
| MeSH | D017248 |
| PubChem CID | 123130 |
| RTECS number | XM8225000 |
| UNII | F7H7X8K29P |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | UFC8VC4LU0 |
| Properties | |
| Chemical formula | C4H5NOS |
| Molar mass | 129.17 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Odorless |
| Density | 1.33 g/cm3 |
| Solubility in water | soluble |
| log P | 0.06 |
| Vapor pressure | 1.6 hPa (25°C) |
| Acidity (pKa) | 13.1 |
| Basicity (pKb) | 14.98 |
| Magnetic susceptibility (χ) | -60.0·10^-6 cm^3/mol |
| Refractive index (nD) | 1.595 |
| Viscosity | 1.198 mPa·s (25°C) |
| Dipole moment | 2.11 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 117.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -51.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –2144 kJ·mol⁻¹ |
| Hazards | |
| GHS labelling | GHS02,GHS07 |
| Pictograms | GHS06, GHS08 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements for 5-Thiazolylmethanol: "P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 100°C |
| Lethal dose or concentration | LD50 (oral, rat): 370 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 5-Thiazolylmethanol: **380 mg/kg (rat, oral)** |
| NIOSH | Not established |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/mL |
| Related compounds | |
| Related compounds |
5-Thiazolecarboxaldehyde 5-Methylthiazole Thiazole 2-Methylthiazole 5-Bromomethylthiazole |