Even before 5-Oxo-DL-Proline earned attention in biochemical circles, the study of amino acid derivatives kept growing alongside new discoveries in protein chemistry. Early syntheses in the 20th century put proline and its oxo analogs at the center of many metabolic explorations. Researchers in post-war Europe started isolating oxoproline from plant extracts and animal tissues. Its discovery in human urine marked a turning point. A pattern started to form—5-Oxo-DL-Proline can pop up both in normal metabolism and under stress or disease. Biomedical scientists, keen to map inherited metabolic errors like pyroglutamic acidemia, saw its diagnostic potential. As chromatography sharpened, pinpointing 5-Oxo-DL-Proline in clinical profiles became easier than ever, spurring researchers to push deeper into its chemical quirks and relevance.
5-Oxo-DL-Proline, often called DL-Pyroglutamic acid, straddles the line between a natural metabolite and a handy laboratory standard. In use, it shows up in biochemistry labs, food technology, and pharmacological research. Its zwitterionic structure lends it a stable performance during protein or peptide work. Every bottle or vial comes as a fine white or off-white powder, often sealed to block out moisture. Some see it as a mere chemical; I see it as a clue in unraveling larger cycles—whether glutathione metabolism or even stress responses in cells.
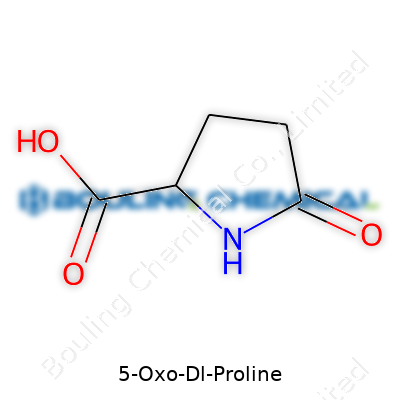
This compound sounds simple, but its balance between acidic and basic groups makes it unique. Its chemical formula is C5H7NO3, and it sits at a molecular weight of roughly 129.1 g/mol. The melting point tends to land right below 185°C when pure, and strong hydrogen bonding shapes much of how it behaves in water. Solubility in both water and ethanol ranks high—a fact that turns it into a reliable player for solutions at many concentrations. The crystalline powder absorbs moisture readily, so tightly sealed storage is best. The ring structure—a five-membered lactam—gives it both rigidity and a spark of reactivity, useful in several chemical tweaks other derivatives won't handle.
Producers outline the chemical name, synonyms (like DL-Pyroglutamic Acid), CAS number, batch code, purity percentage, and suggested storage conditions right on the label. High-purity forms approach or reach 99%, ideal for sensitive analytical work or high-grade pharmaceuticals. Analytical specs typically include loss on drying below 0.5%, minimal heavy metals, and verification by chromatography. Lot and expiry information give enough detail for any lab manager to check back against a database. One practice I've valued—examine the certificate of analysis for little numbers that flag pH or the presence of residual solvents. These matter more than a fancy label.
Lab syntheses often start from L- or DL-glutamic acid, using cyclization under controlled acidic or dehydrating conditions. Heating with acetic anhydride or phosphoric acid reliably triggers ring closure, yielding 5-Oxo-DL-Proline in predictable yields. Some routes work at industrial scale, focusing on solvent reuse and temperature control for efficiency. Afterwards, filtration and crystallization take center stage. Any industrial chemist keeping an eye on cost, waste management, and product purity will respect how seamlessly these production routes have evolved from flask-scale experiments to kilo-scale batches.
5-Oxo-DL-Proline stands out for how willingly it participates in peptide coupling and esterification reactions. Laboratories use it as a building block for protected peptides or even as a chiral pool material for synthesizing tailored analogs. The free carboxyl group makes ester formation easy, supporting all sorts of research into prodrugs or enzyme inhibitors. In strong acids or bases, it hydrolyzes to proline or linear glutamic acid. N-acylation and methylation represent common derivatizations, opening the door to novel uses in drug or material science. If you ask chemists, they'll recall using this molecule to tinker with the hydrophobicity or bioactivity of larger peptides—not just for curiosity, but for tangible medicinal purposes.
The compound earns several monikers, reflecting distinct contexts: DL-Pyroglutamic acid, DL-2-Pyrrolidone-5-carboxylic acid, and DL-PGA. Product catalogs may list it under EINECS 200-293-7 or simply as Pyroglutamate when describing its salt forms. The synonyms can confuse, but a quick check for the CAS number—89-00-9—clears up most questions for procurement teams. Commercial labels may stress “DL” when it comes as a racemic mix, since single-enantiomer versions lag in ready availability and run higher in price.
Laboratories monitor dust levels since the fine powder can irritate eyes or the respiratory tract. Basic gloves, goggles, and good air flow suit routine use. Classified at low hazard, 5-Oxo-DL-Proline still asks for respect—especially in bulk handling or when dissolving in strong acids or bases, which spike risk. Storage places should stay cool and dry. Spillage on benchtops cleans up easily with water or dilute base, but avoid washing large amounts into drains—municipal water treatments do not handle high chemical loads gracefully. From personal experience, a messy spill usually means more paperwork than danger, but rushing cleanup helps no one. Most technical data sheets reference OSHA and EU Reach guidelines, making regulatory compliance straightforward.
5-Oxo-DL-Proline turns up in protein sequencing, chromatography standards, and as a model substrate for enzyme studies. Biochemists value it for simulating metabolic cycles, researching glutathione depletion, and developing diagnostics for inherited metabolic diseases. In food, its derivatives serve as stabilizers or water-binders. Some pharmaceutical companies work with its derivatives to shape controlled drug release or improve water solubility. My exposure to analytical chemistry showed it serves as a solid anchoring point, sharpens standard curves, and helps calibrate HPLC and amino acid analyzers. Peptide chemists reach for it to cap N-termini or tweak molecular interactions.
R&D teams worldwide continue to tease out its roles in pathologies tied to oxidative stress, brain metabolism, and renal function. Neurologists link its elevated levels to cognitive decline or central fatigue, prompting clinical interest. Enzyme kinetics relying on 5-Oxo-DL-Proline fuel insights into recycling pathways such as those involving 5-oxoprolinase. Drug development lines investigate novel analogs with improved bioavailability and metabolic stability. Spin-off areas probe biodegradable polymers and cryoprotectants, sometimes starting from pyroglutamic acid structures as source material. Time in a university lab taught me how molecules like this can trigger new thinking—one assay or pilot project at a time.
Toxicological profiles for 5-Oxo-DL-Proline show low acute oral and dermal toxicity in mammals. Chronic exposure at high concentrations can increase the risk of metabolic acidosis due to buildup in the glutathione cycle. Rodent studies report minimal systemic toxicity at regular laboratory doses, yet repeated exposure underscores the importance of monitoring metabolic intermediates and pH balance. Cell assays test for cytotoxic effects in neurons, kidneys, and liver cells, with most findings supporting safety at realistic usage levels. Any substance impacting central metabolic cycles deserves close scrutiny. Clinical experience with pyroglutamic acidemia, though rare, highlights the need for medical awareness, especially among patients with compromised renal function or existing metabolic challenges.
Looking ahead, wider data-sharing will support safer uses and deeper explorations. The healthcare field eyes 5-Oxo-DL-Proline as a potential biomarker for oxidative stress or as a future component in oral supplements designed to tweak neurochemistry. Analytical chemistry keeps finding new ways to use it as an internal standard or quality-control marker. Materials science hints at bio-inspired polymers made from pyroglutamate backbones, aiming for biodegradable and nontoxic films or coatings. If funding follows, new diagnostic assays could track subtle shifts in metabolic health. My bet: as basic researchers and clinicians cross paths more often, they will find even broader medical, nutritional, and industrial niches for this versatile small molecule. Smart regulation and ongoing toxicity evaluations will keep its potential benefits front and center, supporting care for both people and the environment.
5-Oxo-DL-Proline isn’t a name that comes up at the grocery store, but it plays a quiet role in science labs and medicine cabinets. This chemical, which scientists also call pyroglutamic acid, pops up regularly in research looking at the way our bodies process proteins and maintain health. If you’ve worked any time in a biology lab, you’ve seen 5-Oxo-DL-Proline on the shelves or listed in catalogues, mostly used for studying amino acid pathways or as a building block for more complicated research.
Researchers look at 5-Oxo-DL-Proline in studies on metabolism. This molecule sits at a key intersection in the breakdown and recycling of proteins, and here's why that matters: getting protein metabolism right means keeping cells healthy. Medical labs test for levels of pyroglutamic acid in patients to catch metabolic problems—if these acid levels go too high, doctors start looking for clues about liver issues, nutritional deficiencies, or inherited metabolic disorders. Hospitals use urine analysis to flag metabolic syndromes, and 5-Oxo-DL-Proline makes the list of substances they check.
Pharmaceutical companies use 5-Oxo-DL-Proline as more than a subject of study. It helps design drugs, often acting as a stabilizer or precursor in synthesis. Some medicines, especially those tackling memory loss and cognitive issues, rely on molecules closely related to 5-Oxo-DL-Proline. The reasoning here lines up with how our brains work: neurons depend on the balance of small molecules. Disruptions lead to problems, and scientists chase stable versions to restore it. Consider medications for cognitive support—some of these tap into 5-Oxo-DL-Proline or its derivatives. While these drugs promise benefits, more research must confirm safety and effectiveness. Patients depend on clear evidence, not marketing claims.
Occasionally, 5-Oxo-DL-Proline appears in the food industry for flavor enhancement. This reminds me of how tiny tweaks in a recipe can dramatically shift taste. Manufacturers have used compounds related to pyroglutamic acid to boost savory notes, especially for processed foods. Even though its main home stays in labs, knowing that it can turn up in processed foods matters, especially for anyone keeping a close count on what they eat due to sensitive stomachs or rare metabolic conditions.
5-Oxo-DL-Proline grabs the attention of scientists because of what it tells us about our bodies. Metabolites like this give researchers a glimpse into the complex story of health and disease. Testing for this compound brings concrete benefits in diagnosing rare diseases, especially in kids with unexplained symptoms that baffle general tests. Accurate measurement saves time, money, and sometimes lives. Still, lessons learned over years in clinical labs teach us to treat test results with care—not every high or low reading translates to illness, and context matters.
One major challenge comes from a lack of standardized guidelines in interpreting pyroglutamic acid results. Doctors benefit from more training and better awareness of metabolic pathways. Federal agencies and research bodies can help by setting good standards for test quality and data comparison. As scientists continue to map out the uses and implications of 5-Oxo-DL-Proline, connecting this knowledge to better treatments stands as the true measure of its value. Collaboration between labs, hospitals, and regulators will help clear up confusion, translating laboratory findings into action for real people.
5-Oxo-DL-Proline, also known as pyroglutamic acid, exists in small amounts in the human body, as part of our normal metabolic processes. It plays a role in the gamma-glutamyl cycle, closely linked to how we produce and recycle glutathione. Glutathione stands out as one of the body's most potent antioxidants, helping fend off oxidative stress and supporting liver function. Some people look to 5-Oxo-DL-Proline supplements, thinking they might boost mental clarity, reduce fatigue, or play other helpful roles in the body.
Large-scale research on the direct safety of 5-Oxo-DL-Proline as a supplement in humans remains scarce. Most of what we understand comes from its natural occurrence and from animal studies. At typical dietary and laboratory levels, it shows low toxicity. Our bodies can break it down and clear it out, especially when our metabolic systems run smoothly. Sometimes, hospitals test for high levels of this compound to help diagnose rare metabolic disorders, which can occur when normal breakdown pathways get blocked, usually by underlying health problems or medication reactions.
I dug into available clinical reports, looking for any signals of trouble from supplementing or eating higher amounts of 5-Oxo-DL-Proline. There’s little information about major problems from regular food sources or in healthy folks. The risk rises only when the body’s regular processing fails. For example, children with inborn metabolic conditions, or adults with kidney or liver failure, sometimes show dangerous buildups.
The supplement world often outpaces scientific research. Despite rising interest in nootropics and metabolic boosters, regulatory agencies haven’t labeled 5-Oxo-DL-Proline as “generally recognized as safe” for large, long-term doses. Most people ask, “Should I try it if I want to boost my mental energy?” Without strong human data, the answer sits in a gray area.
In my experience, it pays to remember that just because a compound turns up naturally in the body doesn’t mean it’s risk-free in large, concentrated doses. Many natural chemicals go from helpful to harmful with a bigger dose or when combined with other substances. Given that little is known about how 5-Oxo-DL-Proline supplements might interact with prescription medications, the safety net shrinks for those looking to try it as a daily booster.
To move forward safely, clearer and longer-term studies need to happen. Researchers could test supplement doses in healthy volunteers and in vulnerable groups, with careful tracking of kidney and liver function. Until more data comes in, I suggest caution—especially for anyone with compromised kidney or liver health, or anyone taking medications known to mess with amino acid metabolism.
Doctors and registered dietitians can play a key role. Sharing detailed medical histories and supplement use open the door to safer choices. Quality testing by reputable brands also matters, since supplements sometimes contain unexpected fillers or incorrect dosages.
Based on current research and personal experience in healthcare, 5-Oxo-DL-Proline doesn’t raise big red flags in healthy bodies at typical levels. Still, nobody has proven its safety at high supplemental doses for the long term. Those considering supplements should talk with a medical professional who looks beyond marketing claims and weighs individual health factors.
5-Oxo-DL-Proline shows up in a lot of places—labs, supplements, and the bigger world of amino acid research. This compound, also called pyroglutamic acid, hooks into the body’s glutathione cycle. Glutathione acts as a major antioxidant, which means this molecule jumps into some of the body’s most important chemical processes. Because of this, researchers pay close attention to its effects, especially any negative ones.
Pharmacologists have spent years studying derivatives of proline, like 5-Oxo-DL-Proline. Reports sometimes mention headaches, dizziness, or gastrointestinal problems. Folks with kidney or liver conditions don’t clear this compound as easily, which can mean a bigger buildup and riskier side effects.
Doctors know that 5-Oxo-DL-Proline sometimes causes excess acid in the body—a trouble called metabolic acidosis. Medical journals link this to symptoms like confusion, deep breathing, and muscle twitching. Hospitals have seen patients, often those with chronic illness, run into elevated 5-Oxo-DL-Proline levels after certain medications (think acetaminophen) or metabolic stress. Scientists measure plasma pyroglutamic acid during these events and connect spiking levels to worsening symptoms.
High concentrations don’t tend to show up in healthy people. If you have health challenges or use specific drugs, your risk climbs. This pattern isn’t guesswork—labs and clinics have documented it through direct patient monitoring.
Molecules like 5-Oxo-DL-Proline remind us the body’s chemistry moves fast and sometimes slips off track. If something blocks the glutathione cycle, these molecules back up and cause problems. In my own work with nutrition and biology, tracking unexpected side effects from supplements has sometimes pointed people in the right direction. Even a seemingly “natural” molecule holds power to tip someone’s system off-balance, especially if they already have health hurdles.
Most folks outside biochemistry don’t think about pyroglutamic acid until trouble starts—maybe a friend taking new medication suddenly feels unwell, or a lab test hits a weird number. Doctors then dig deeper and sometimes find elevated 5-Oxo-DL-Proline at the core.
Information stands as the most practical defense. People with kidney or liver trouble should talk with their care team before trying new supplements, especially those that touch the glutathione cycle. Avoiding acetaminophen overdoses, drinking enough water, checking medication combinations—all these steps protect your body from building up excess 5-Oxo-DL-Proline.
Professionals can run tests if someone has unexplained confusion, fatigue, or deep breathing—catching the cause and guiding the next steps. They know pyroglutamic acid sometimes plays a role and have tools to measure its levels. Keeping up solid research, sharing real results, and reporting side effects keep everyone safer.
If you’re thinking about using supplements linked to this compound, take time to read up on safety, check in with your doctor, and stay alert for changes. Even basic steps like monitoring hydration or double-checking prescriptions make a difference. Watching how your body reacts and getting care early can turn a scary side effect into a routine fix.
5-Oxo-Dl-Proline often shows up in laboratories and chemical storerooms because of its unique place in research. It has an established profile in biochemistry and can show some quirks when not treated right. Drawing from years spent around similar materials, there always seems to be a story about small neglect leading to big headaches. Storage slips often create more work than careful management at the start.
It’s tempting to set aside containers and move to the next experiment, but high-purity chemicals don’t take care of themselves. 5-Oxo-Dl-Proline can absorb moisture over a few weeks if left in humid rooms. Clumping, degradation, or even loss of integrity results, wasting effort put into sample prep and causing inconsistent research outcomes later. Reliable results demand reliable storage.
There’s no big secret in protecting sensitive chemicals. Airtight glass bottles or well-sealed plastic containers work much better than leaving powders in folded bags. Every experienced lab worker recognizes the difference real lids make compared to improvised covers. Silica gel packs, commonly tossed into vitamin bottles, pull moisture away and keep the inside dry. Tossing a few inside the storage box can help keep the powder free-flowing.
Temperature matters more than many folks realize. Cool rooms slow down slow chemical changes that quietly ruin samples over time. At room temp, many powders keep their stable form for months, but bumping them into a fridge—away from food or strong-smelling reagents—adds another safety edge. Chemical suppliers often recommend 2-8°C as a good range, offering an extra buffer in busy labs where doors open and close all day. Make sure to keep the container closed right after use, since repeated opening lets in air and, with it, water vapor.
Accurate labels save hours of future confusion. A scribbled name on a cap quickly fades or smears. Proper chemical storage includes noting the full name, date received, and—since some suppliers do vary in purity—the manufacturer’s details. This helps any future user gauge how fresh the material is and compare it to original certificates. Contamination creeps in mostly through careless handling, so scoops and spatulas should stay dry and only go into one bottle at a time. Using the oldest batch first keeps stock fresh and avoids a pile-up of forgotten leftovers.
Lab supervisors and safety managers care about consistency, reliability, and safety records because experience teaches hard lessons about shortcuts. Stories travel fast through research teams about a contaminated bottle that ruined a whole set of samples or an unlabelled jar that made even seasoned chemists hesitate. Clear, methodical storage habits reflect on the integrity and reputation of a lab. That trickles back to funding bodies, publication success, and future opportunities for everyone involved.
It’s often just common sense, reinforced by industry guidelines and direct manufacturer recommendations. Handle 5-Oxo-Dl-Proline with regular, careful habits: sealed containers, cool storage, and clear labels. Taking those extra steps pays off in safer, less wasteful, and more dependable research—something everyone can stand behind.
People often look at supplements for answers, hoping to get an edge with their health, performance, or mental sharpness. 5-Oxo-Dl-Proline doesn’t get the headlines like vitamin D or omega-3, but conversation around it keeps coming up. Anyone considering trying it will want real answers, not hype or promises. Questions about dosing always rise to the top—what amount is safe, and what’s smart to take?
Finding a reliable dosage isn’t as straightforward as grabbing advice off social media. Evidence matters. For 5-Oxo-Dl-Proline, published information stays pretty slim, mainly because it isn’t among the nutrients with decades of clinical trials. Researchers know that 5-Oxo-Dl-Proline comes from the body’s natural cycles for glutathione—a key antioxidant. At the same time, manufacturers produce supplements that deliver a much higher dose than the trace amounts people get from diet alone.
Some published human research exists. For example, clinical trials looking at cognitive enhancement or mental fatigue have used doses between 100 mg to 500 mg daily. No reputable study calls for mega-doses or long-term use without monitoring. I learned this myself while scouring research databases—none of the respected sources, including the National Institutes of Health, list a recommended daily intake for this compound. It signals that people using it are in largely uncharted territory, and that drug-like doses bring risks alongside the curiosity factor.
Every time I read up on a supplement, the same rule stands out: safety tops everything. Those with kidney or liver conditions should be extra careful with amino acid analogs like 5-Oxo-Dl-Proline, because the body has to process and break them down. Although a few studies reported no acute toxicity at lower daily dosages (100 mg or less), there just isn’t enough long-term data. Reports on side effects remain rare, but that could be due to the limited pool of users and published studies.
If someone asked my opinion, I’d say start with the lowest possible dose if they’re intent on giving 5-Oxo-Dl-Proline a try—no more than 100 mg daily. Make sure a healthcare provider knows, especially for anyone on medication or managing chronic health problems. Trust in lab-verified quality for any supplement, not mystery compounds online.
Doctors and clinical pharmacists have the background to help watch for early signs of trouble, including reactions with other supplements or drugs. Since the industry isn’t strictly monitored, contaminants and dose inconsistencies do happen more than people expect.
To reach a real consensus on the right dosage, researchers need bigger, longer studies. Until then, claims about brain-boosting or recovery benefits stay speculative. If anyone wants the benefits found in small trials, sticking to the same amounts—around 100 to 500 mg, for short periods under professional guidance—makes the most sense. No supplement can replace good nutrition, daily movement, and enough sleep.
Curiosity pushes the science of nutrition forward, but caution protects health. It’s worth questioning claims, reading every label, and asking about the evidence behind every scoop or pill—especially for something as little studied as 5-Oxo-Dl-Proline.

| Names | |
| Preferred IUPAC name | 5-oxopyrrolidine-2-carboxylic acid |
| Other names |
5-Oxopyrrolidine-2-carboxylic acid Pyroglutamic acid Pidolic acid Pyrrolidone carboxylic acid PGA |
| Pronunciation | /faɪv ˈɒk.soʊ di ɛl proʊˈlaɪn/ |
| Identifiers | |
| CAS Number | 4042-36-8 |
| Beilstein Reference | 89348 |
| ChEBI | CHEBI:28384 |
| ChEMBL | CHEMBL40437 |
| ChemSpider | 12319 |
| DrugBank | DB00111 |
| ECHA InfoCard | 05f180a8-ec16-402d-90d1-c25b266aafd5 |
| EC Number | 3.5.2.9 |
| Gmelin Reference | 5437 |
| KEGG | C00467 |
| MeSH | D017379 |
| PubChem CID | 730 |
| RTECS number | TC7125000 |
| UNII | J6Z7L0J9U8 |
| UN number | 2811 |
| Properties | |
| Chemical formula | C5H7NO3 |
| Molar mass | 129.11 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.546 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.4 |
| Vapor pressure | 0.0000206 mmHg at 25°C |
| Acidity (pKa) | 3.32 |
| Basicity (pKb) | 2.89 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.510 |
| Viscosity | 1.27 g/cm³ |
| Dipole moment | 12.8881 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 143.6 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | -652.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1142.8 kJ/mol |
| Pharmacology | |
| ATC code | N02CX10 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | `CN1C(=O)CCC1=O` |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: "P261, P305+P351+P338 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 143°C |
| Autoignition temperature | 410°C |
| Lethal dose or concentration | LD50 Oral - Rat - 6,800 mg/kg |
| LD50 (median dose) | LD50: 9400 mg/kg (rat, oral) |
| NIOSH | NJ0135000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 20 mg/kg |
| IDLH (Immediate danger) | There is no specific IDLH (Immediate Danger to Life or Health) value established for 5-Oxo-DL-Proline. |
| Related compounds | |
| Related compounds |
Pyroglutamic acid L-Proline Glutamine Glutamic acid 2-Oxoglutaric acid |