5-Nitrothiazol-2-ylamine, sometimes written as 2-Amino-5-nitrothiazole, tells a fascinating story that goes back to the days when chemists started to unlock the secrets of heterocyclic compounds. Thiazole-based molecules became a hot topic in the early 20th century, mostly due to their antimicrobial properties and promise in medicinal chemistry. Not long after the isolation of thiazole itself, tweaks and substitutions gave rise to a wave of derivatives. The nitro group at the five position and the amine at the two position transformed this compound into a research staple for both medical and industrial chemists. Decades ago, research in sulfa drugs and nitrothiazole derivatives brought this chemical into the spotlight as part of the search for reliable agents to tackle infections and parasites in both humans and animals.
5-Nitrothiazol-2-ylamine stands out for versatility. Its molecular formula—C3H3N3O2S—might look simple, but this chemical serves as a backbone in many syntheses, including drug intermediates and specialty reagents. The solid, yellow-to-orange crystalline powder form quickly tells you it isn’t just another benchtop extra—many laboratories depend on its reliability and distinct chemical reactivity. The structure boasts both electronic complexity and a well-positioned amine for coupling or further modification. People often talk about it as a starting material for designing new drugs, pesticides, and dyes.
Physical traits, such as melting point, color, and solubility, steer both handling and application. This compound generally melts around 210°C, indicating a stable molecular lattice. Water solubility lands in the moderate range and increases in polar organic solvents, so it's easy to handle in both lab and small-scale manufacturing settings. It does not smell much, but the vivid color can stain surfaces easily, a headache familiar to those working with related nitro compounds. The molecule’s nitro group draws special attention, acting as a strong electron-withdrawing moiety, making the thiazole ring more susceptible to nucleophilic attack in certain reactions or as an activating point for cyclization strategies.
Most reputable suppliers provide certificates of analysis listing assay often above 98%. You want careful packaging—amber glass vials or HDPE containers guard against light and moisture. Librarians of chemical storage advocate clear GHS labeling with the signal word “Warning” due to potential hazards present with nitro aromatics. Labels highlight important facts: chemical identity, batch number, purity, hazard pictograms, and the supplier’s contact. Product sheets usually mention melting point, storage conditions (cool, dry, well-ventilated space), and handling instructions to avoid skin or inhalation contact. It’s not just regulatory—anyone who's ever spilled nitro-compounds knows careful labeling keeps accidents down and workflows smooth.
Laboratory synthesis generally starts from 2-aminothiazole, a building block found in many chemical catalogs. Nitration using a mixture of concentrated nitric acid and sulfuric acid at controlled temperatures replaces a hydrogen atom at the 5-position, giving 5-nitrothiazol-2-ylamine. The reaction can be hazardous; careful temperature control and slow addition of reagents—along with good ventilation—reduce the risk of runaway exotherms or the formation of nitrogen oxides. Filtration and recrystallization in ethanol or water give a product pure enough for most syntheses, although some labs choose further chromatographic purification for electronic applications or high-sensitivity studies.
5-Nitrothiazol-2-ylamine’s chemistry revolves around functional group manipulations. The free amino group lets researchers participate in acylation, diazotization, or Mannich reactions, opening doors to a variety of pharmaceutical intermediates. The nitro group serves as both a synthon and a modifiable handle—reduction produces diamino derivatives, which are crucial in dye manufacture. Other researchers use the nitrothiazole to build more elaborate fused-ring systems by condensation, microwave-assisted cyclizations, or tandem reactions. Experienced hands, especially in medicinal chemistry, use these reactions to expand molecular diversity, especially to target antimicrobial or anticancer activity.
In catalogs and scientific publications, 5-nitrothiazol-2-ylamine pops up under various names such as 2-Amino-5-nitrothiazole, NSC 39738, and Nitazol. Certain veterinary formulations prefer the trade name “Thiazamide.” Pharmaceutical reference works sometimes shorten the label simply to “Nitrothiazole.” Having worked in both industry and academia, I know inconsistent naming can mess up an order or confuse students, so double-checking CAS number 121-66-4 and structural diagrams keeps errors away.
Working safely with nitrothiazoles means wearing gloves, goggles, and full-length lab coats. Risk isn’t just talk—acute toxicity via ingestion or inhalation means spills and dust clouds need cleaning up right away. Local exhaust ventilation or fume hoods dramatically cut exposure. Some regulations (OSHA, REACH) call for risk assessments, training, and PPE documentation. Material Safety Data Sheets (MSDS) regularly update on hazard codes, emergency response, and storage recommendations. In my lab days, storing away from strong reducing agents and acids kept reactions safe. Regular audits check for container integrity and proper inventory logging. People who cut corners can pay dearly with dangerous exposures, lost batches, and even regulatory action.
The practical reach of 5-nitrothiazol-2-ylamine covers pharmaceuticals, agrochemistry, dyes, and, in some cases, research reagents. Its base skeleton forms part of drugs targeting protozoal infections like Giardia or Trichomonas. Some countries register nitrothiazole derivatives as antimicrobials in veterinary science. Chemists use it to screen for new enzyme inhibitors and potential cancer therapeutics. Dyes and pigments owe vivid, stable coloring to the aromatic nitro group’s high chromophoric activity, a fact exploited in textile industries seeking bright, enduring colors. My own work once intersected with agricultural chemistry, exploring how nitrothiazoles fend off plant pathogens in field conditions. That field-level work made it obvious: real-world applications tie lab molecules into daily life.
Researchers continue to discover new targets for nitrothiazol-2-ylamine. A major thrust focuses on overcoming drug resistance in parasites and bacteria. Scientists design analogs with tougher activity against resistant strains, sometimes tweaking the side chains or fusing additional rings. Green chemistry also shapes newer synthetic pathways—microwave irradiation and solvent-free reactions promise faster production with less waste. In the world of diagnostics, researchers link this molecule to fluorescent or affinity tags for sensitive biosensor development. Investment in medicinal chemistry pipelines ensures that the next generation of nitrothiazoles will meet both efficacy and safety standards.
Every strong chemical brings safety questions. Studies consistently report that 5-nitrothiazol-2-ylamine causes moderate acute toxicity, mostly to the liver and kidneys, at high doses in animal testing. Researchers work to define safe therapeutic dosages and identify metabolic by-products, some of which can be more toxic than the parent compound. Breakdown under light or in soil usually forms less active species, but some environmental persistence raises questions among regulators. Laboratories and manufacturers invest time and money in proper waste treatment, neutralization, and environmental controls so that traces don’t end up in water supplies or food.
The journey doesn’t stop here. Synthetic modifications, improved green production, and digital modeling unlock new uses every year. AI-guided drug design points to unprecedented precision for targeting tough-to-treat infections and cancer cells. Environmental scientists keep an eye on the molecule’s footprint, pushing for safer handling and less persistent by-products. In the end, both experienced chemists and eager newcomers look to further refine this versatile molecule, promising safer drugs, smarter dyes, and better tools for science worldwide.
Some compounds don’t often show up on the news, but 5-Nitrothiazol-2-Ylamine shapes more parts of daily life than most folks realize. I remember working in a small lab where every reagent mattered. If someone ran out of a single intermediate, the whole process stalled. One bottle had a label: 5-Nitrothiazol-2-Ylamine. It was one of those chemicals the older chemists always kept an eye on. It seemed like whenever a project turned tricky, this compound helped push things forward.
Pharmaceutical development often feels like a puzzle. Drugs need building blocks that not only fit a reaction but also lead to life-changing medicines. 5-Nitrothiazol-2-Ylamine takes a crucial role here. Medicinal chemists count on it as a starting point for several types of antimicrobial and antiparasitic agents. Many nitrothiazole-based drugs target tough infections that antibiotics can’t clear up. The broad spectrum seen in these molecules roots back to this very chemical.
One of the recognizable drugs, nitazoxanide, owes its existence to chemistry involving compounds like 5-Nitrothiazol-2-Ylamine. This medication clears up protozoal infections and helps treat diseases that hit hardest in places with limited healthcare. Growing up in an area where intestinal parasites hit families year after year, I saw how one new medicine changed outcomes. Watching pharmacies finally stock something that worked felt like a small victory in an old fight.
Beyond infectious diseases, researchers lean on this compound in cancer studies. Scientists study nitrothiazole derivatives for their ability to disrupt cell growth in certain tumors. The logic here is simple: start with a molecule which has already shown the ability to break down stubborn microbial defenses, and adjust it to go after cancer pathways. Every year, new journal articles spring up connecting analogues of 5-Nitrothiazol-2-Ylamine to promising lab results.
Drug discovery depends on accessible intermediates like this. Without reliable sources, researchers spend weeks making starting materials instead of testing new ideas. Better supply lines mean less wasted effort, letting scientists devote more hours to breakthroughs.
Chemical synthesis forms the backbone of countless industries. Agrochemical companies use variations of nitrothiazole derivatives to create treatments that protect crops. Farmers count on these innovations to battle crop-killing parasites and diseases, helping entire communities avoid famines. My uncle’s soybean farm once lost half its yield when leaf blight swept through. After newer protectants reached the market, losses dropped, bringing relief and stability. These innovations often start in research benches with familiar names like 5-Nitrothiazol-2-Ylamine.
With all its uses, 5-Nitrothiazol-2-Ylamine brings up hard questions too. Some nitro compounds could cause environmental concerns if handled carelessly. Proper lab protocols and guidelines keep spills rare, but tight oversight remains important. Companies and labs that treat every link in the supply chain responsibly help protect neighborhoods and the environment alike. Regulatory frameworks, careful storage, and waste management shape the difference between innovation and accident.
In my work, I’ve learned that pushing science forward comes with balancing discovery with safety. Open sharing of best practices and stronger relationships between researchers, producers, and regulators lead to breakthroughs everyone can trust.
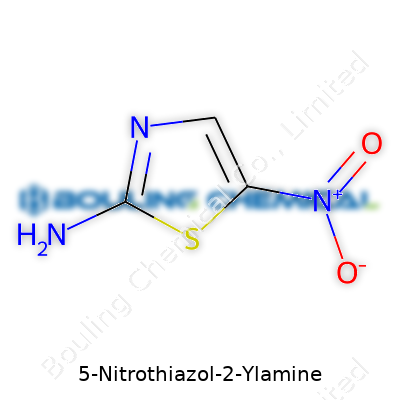
Chemical compounds don’t always get a lot of time in the spotlight, but compounds like 5-Nitrothiazol-2-Ylamine tell a pretty interesting story. Once you look at its molecular structure, you spot the fused ideas from organic chemistry: sulfur and nitrogen together in a five-membered ring, along with a nitro group sticking out on one side and an amino group on the other. The thiazole ring forms the backbone, and chemists see the barcode of its chemical formula—C3H3N3O2S.
I remember stumbling over the drawing of thiazole rings back in undergraduate labs, pencil smudges all over my notes because I kept tracing out those five-membered rings with a sulfur and a nitrogen in the right places. 5-Nitrothiazol-2-Ylamine features a nitro group bound at the 5-position and an amino group attached at the 2-position of the thiazole ring. The nitro group (NO2) pulls electrons, while the amino group (NH2) does the opposite. It changes how this compound interacts with enzymes, cell membranes, and other chemicals in experimental settings.
The actual molecular structure does not leap out to everyone, but picture this: a compact five-pointed ring, sulfur at one angle, nitrogen at another, and carbon filling out the rest. At carbon number 5, the nitro group extends away from the ring, coloring the molecule's properties with polarity and reactivity. At carbon number 2, an amino group attaches, lending potential for hydrogen bonding. The formula C3H3N3O2S reads as a short story of three carbons, three hydrogens, three nitrogens, two oxygens, and a sulfur atom.
Why bother over such detail? Plenty of drugs, dyes, and agricultural agents pull their punch thanks to nitrogen-sulfur rings, and swapping in a nitro or an amino group can tune solubility, biological activity, and toxicity. Nitrothiazoles have a tough reputation, showing up in certain antibiotics and antiprotozoal drugs. The arrangement of functional groups controls binding in the active sites of enzymes, and even a shift in position can knock out effectiveness or amp up side effects.
Researchers value 5-Nitrothiazol-2-Ylamine because of its potential as a building block. Modifying its structure has led to compounds that block pathogens, act as intermediates for more complex molecules, and give chemists a way to test their synthetic techniques. Chemical safety always comes along for the ride. Nitro-containing compounds can be sensitive, and proper handling in the lab means working under a hood, gloves, and careful recordkeeping.
Keeping up with the science means drawing on reliable sources. The structure and formula of 5-Nitrothiazol-2-Ylamine have been written up in peer-reviewed chemistry journals. PubChem and other databases back up this structure—no need to rely on rumors or sales pitches. Open communication between researchers and the public allows people to check the real shape and significance of a molecule themselves.
If research points toward a use for 5-Nitrothiazol-2-Ylamine—whether in medical, agricultural, or industrial settings—scientists must test not just what it does, but how it behaves long-term. Testing for breakdown in soil, water, and living tissues helps avoid trouble down the road. Collaboration across chemistry, pharmacology, and environmental science keeps these discoveries on track and reduces risks of misuse or mismanagement. Sharing research methods and findings with clear citations becomes a foundation for progress.
Few folks outside the chemistry world have run into 5-Nitrothiazol-2-Ylamine. Those who work with it know the stakes behind proper storage and handling. This compound serves as a building block in research labs, yet improper handling sometimes leads to serious incidents—like surprise spills or worse, chemical fires.
Safety in chemical labs often gets taught as “common sense,” but lab memories tell a different story. It’s not just about locking bottles in a cabinet but understanding why those rules keep accidents from turning a lab into a disaster zone. I once saw a colleague handle a thiazole derivative without double-checking for static discharge. That mistake led to a small fire—and nobody forgot the lesson. That story drives home why safe handling can't rely on luck.
5-Nitrothiazol-2-Ylamine can trigger toxic reactions if it comes in contact with skin or eyes, is inhaled, or gets swallowed accidentally. The nitro group within the molecule means extra care during storage. Nitrogen-rich molecules can break down and release toxic gases if exposed to heat or strong oxidizers. Reports from industrial toxicology studies show that some nitro compounds cause organ damage with repeated low-dose exposure. So, safety isn’t just about immediate burns or rashes—it’s about protecting long-term health.
This compound can also be sensitive to static and friction. Several thiazole derivatives gained notoriety in chemical safety texts because of unexpected reactivity and the hassle of cleaning up after an accidental release.
Storing 5-Nitrothiazol-2-Ylamine properly starts with a dry, cool location—the less sunlight, the better. Direct heat or sunlight can nudge some nitro compounds toward instability. I’ve worked in both old and modern labs, and every reliable chemical storage area shares the same features: solid shelving, tightly-sealed containers, and temperature monitoring. Spill trays underneath storage shelves save time during cleanup and help catch leaks before they spread.
Don’t stick this compound next to acids, oxidizers, or reducing agents. Too many lab accidents stem from haphazard chemical placement. One poorly labeled shelf can start a reaction just by proximity. Organize storage by chemical family, not just alphabetically. It takes a little more time, but it can stop accidents before they start.
Gloves, goggles, and lab coats keep exposure in check. Nitrile gloves work well for most nitro compounds. I always double up when possible, especially if I notice a tear. Use a fume hood instead of open benches. Tiny particles and vapors can cause real harm, even if you can’t see them. Small steps, like airing out the workspace or keeping containers shut tight, stack up to make a big difference.
If something spills, don’t grab paper towels—reach for spill kits made for chemical hazards. Neutralizing agents and appropriate absorbents make a cleanup less risky. Dispose of waste through designated programs. Mixing it with other lab trash is a shortcut to trouble.
Years of lab work taught me that safe practices with 5-Nitrothiazol-2-Ylamine don’t just protect individual researchers—they keep the whole workplace running. Good training and a team culture where people call out sloppy storage or handling stops serious incidents before they start. Experience shows that the best labs don’t focus only on research—they respect the chemistry and the risks by turning caution into habit, every time.
5-Nitrothiazol-2-ylamine, a compound that turns up in labs and some industries, doesn’t exactly have a reputation you’d call gentle. This chemical has enough punch to push researchers and safety managers to take it seriously. ECHA sources put it in the category of substances that can irritate eyes, skin, and the respiratory tract. It’s not unreasonable to assume breathing dust or fumes from this chemical stirs up coughing, throat irritation, and discomfort. Touching it with bare hands or letting it splash on skin can trigger dermatitis or worse, depending on one’s sensitivity.
On the toxicity front, thiazole derivatives—especially those with nitro groups—sometimes show up in research as having mutagenic or cytotoxic effects. No one wants to gamble with potential long-term risks to DNA. Chemicals with these features often attract regulatory oversight, for very good reason. If inhaled, tiny particles of this powder can work into the lungs, and based on what’s observed with structurally similar compounds, this triggers inflammation or chemical pneumonitis. Not the sort of thing you want recurring through regular lab days.
The nitro group catches attention not just for health, but also for ignition danger. Chemicals with a nitro tag tend to be a fire risk. Mishandling, static sparks, or heat can turn a routine mixing job into a scorched mess fast. Accidental spills near drains drag in another worry: the environmental impact. Thiazole rings stay persistent in soil and water, posing a risk to aquatic life. Just a bucket or two down the wrong pipe, and the local river life gets a hit it doesn’t recover from quickly.
Anyone handling this compound in a lab or warehouse can tell stories about uncomfortable rashes or persistent coughs after a careless moment. Reliable personal protective gear cuts down those stories. Nitrile gloves, long sleeves, and eye protection form a basic suit of armor. Respirators get rolled out if the air could turn dusty or if volatile fumes come up on heating. That means simple cloth masks don’t make the cut—fit-tested, certified respirators do.
Working under good ventilation matters. Fume hoods keep airborne particles from floating into unsuspecting lungs. Forgetting to double-check the hood fan or skipping glove changes is where most seasoned chemists see the worst mistakes. Always label containers, skip decanting over open benches, and store the powder far from direct sunlight, flames, or acids. Anyone who’s seen a chemical spill spread through a cluttered workspace knows one mishap creates hours of emergency cleanup.
Throwing chemical waste in the regular bin brings trouble for janitors and for anyone downstream, literally. Proper disposal goes through a hazardous waste stream, never into the municipal trash or the sink. Contaminated rags and gloves belong in sealed bags, collected by licensed handlers.
Every time a team runs safety drills or shares near-miss stories, it’s not just a formality. It’s a reminder that the difference between a routine day and a health incident often rests on small decisions. Real experts learn from the ugly surprises, track regulations from OSHA and ECHA, and make sure their team knows the risks without sugarcoating.
As a rule I follow: never trust a chemical with a nitro group to “sit quietly” on the shelf. Clear procedures, buddy checks, and a culture willing to speak up if something looks off keep accidents rare. It’s not just about compliance; it’s about protecting health, careers, and the environment where every decision today plays out for years down the line.
The search for high-purity 5-Nitrothiazol-2-Ylamine brings up a series of decisions and responsibilities. Every lab researcher knows that finding chemicals with real reliability gets complicated for reasons beyond price. Sourcing this compound takes more than just typing a product code into a search engine.
I remember working late in the chemistry building, scanning catalogs from companies like Sigma-Aldrich, TCI, and Alfa Aesar. Ordering from a reputable supplier always came first because low-grade material wastes time, money, and trust in your results. High-purity 5-Nitrothiazol-2-Ylamine isn’t sitting on every shelf, so checking site accreditation should be part of your process.
Not every supplier offers purity specs, batch analysis, or solid documentation. Genuine chemical suppliers show a COA (Certificate of Analysis), MSDS (Material Safety Data Sheet), and batch test results. Sites with ISO certification and transparent records protect your project and career.
In recent years, chemical regulations got more detailed. Regulatory bodies like the DEA, EU REACH, and national boards want to know exactly who uses certain chemicals. Some compounds tie to pharmaceutical or research risks. You need a registered lab environment, business license, or signed end-user declaration before a shipment even leaves the warehouse.
Attempting to bypass rules—especially on high-purity materials—leads to blocked shipments, fines, or worse. Even after securing your material, reference the MSDS to prevent exposure or environmental spills. Pipetting and weighing goes smoother in a lab with working ventilation, PPE, and safety routines.
Online vendors flood the search results, but it’s risky to buy specialty chemicals through platforms like Amazon or unknown international sites. Several years ago, our lab received a mislabeled “research chemical” from an offshore seller. The label looked off, and batch number didn’t match the COA. Labs relying on questionable suppliers endanger their data, equipment, and people.
Analytical verification fixes nothing after you’ve wasted a week on unusable material. Suppliers with real customer service, references, or industry presence have more to lose than an obscure pop-up storefront. Before making a purchase, check reviews in scientific forums or reach out to colleagues for feedback.
Direct contact with supplier technical teams saves time. They may have alternative grades, ready stock, or safe lead times. Custom syntheses from a CRO (contract research organization) become the next step for unusual purity levels, provided you can justify the expense and timeline.
Some labs, especially in academia, share material or pool orders to lower costs and pass compliance. Informal supplier recommendations travel quickly in close-knit research circles. Local distributor reps sometimes know more about shipping routes and documentation than anyone online.
Sticking with accredited vendors, verifying documentation, and understanding regulations gives you the best shot at secure, steady supply of high-purity 5-Nitrothiazol-2-Ylamine. Modern science depends on reproducibility and trust—in your materials, methods, and safety. Pay attention to small details, because those are what keep experiments on track and reputations untarnished.
| Names | |
| Preferred IUPAC name | 5-nitro-1,3-thiazol-2-amine |
| Other names |
Aminonitrothiazole 5-Nitro-2-aminothiazole 2-Thiazolamine, 5-nitro- 5-Nitrothiazol-2-ylamine |
| Pronunciation | /ˌfaɪˌnaɪtroʊˌθaɪəˈzoʊl.tuːˈæm.iːn/ |
| Identifiers | |
| CAS Number | 3986-57-6 |
| 3D model (JSmol) | `3D model (JSmol)` **string** for **5-Nitrothiazol-2-Ylamine**: ``` CC1=NC(=S)S1N(=O)=O ``` *(Note: This string is in SMILES format, commonly used for JSmol 3D viewers.)* |
| Beilstein Reference | 383608 |
| ChEBI | CHEBI:77885 |
| ChEMBL | CHEMBL414411 |
| ChemSpider | 23563470 |
| DrugBank | DB08702 |
| ECHA InfoCard | 100.022.347 |
| EC Number | 612-062-6 |
| Gmelin Reference | 731211 |
| KEGG | C14360 |
| MeSH | D017382 |
| PubChem CID | 222349 |
| RTECS number | XN8575000 |
| UNII | C8SD4P7ROE |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C3H3N3O2S |
| Molar mass | 158.15 g/mol |
| Appearance | Yellow to orange crystalline powder |
| Odor | Odorless |
| Density | 1.7 g/cm3 |
| Solubility in water | Slightly soluble in water |
| log P | 0.3 |
| Vapor pressure | 6.0E-4 mmHg at 25°C |
| Acidity (pKa) | 9.19 |
| Basicity (pKb) | 5.93 |
| Magnetic susceptibility (χ) | -64.8×10^-6 cm³/mol |
| Refractive index (nD) | 1.736 |
| Dipole moment | 3.85 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 141.3 J mol⁻¹ K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | Std enthalpy of formation (ΔfH⦵298) of 5-Nitrothiazol-2-Ylamine: 81.2 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1720 kJ/mol |
| Pharmacology | |
| ATC code | J01XX07 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P270, P273, P280, P301+P312, P305+P351+P338, P501 |
| NFPA 704 (fire diamond) | 3-2-1-W |
| Flash point | 97.7 °C |
| Lethal dose or concentration | LD50 (oral, rat): 640 mg/kg |
| LD50 (median dose) | LD50: 2100 mg/kg (rat, oral) |
| NIOSH | NA5600000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 2 mg/m3 |
| IDLH (Immediate danger) | NIOSH has not established an IDLH for 5-Nitrothiazol-2-ylamine. |
| Related compounds | |
| Related compounds |
4-Methyl-5-nitrothiazol-2-amine 5-Nitro-2-aminothiazole 2-Amino-5-nitrothiazole 5-Nitrothiazole 2-Methyl-5-nitrothiazole |