Interest in imidazole chemistry goes back more than a century. Early research focused on natural purines, and chemists noticed the unique reactivity of fused imidazole rings in biological molecules. Synthetic modifications became popular in the mid-20th century, as the pharmaceutical and agrochemical industries searched for new building blocks. 5-Methyl-1H-imidazole-4-methanol stood out for its unique methyl and hydroxymethyl substitutions, opening the door to a new set of chemical reactions and potential biological activity. Laboratories in Europe and Japan began publishing methods for its preparation in the 1960s. Thanks to this early groundwork, today's researchers view 5-methyl-1H-imidazole-4-methanol as a versatile, approachable molecule, both for further derivatization and for direct study in biologically relevant systems.
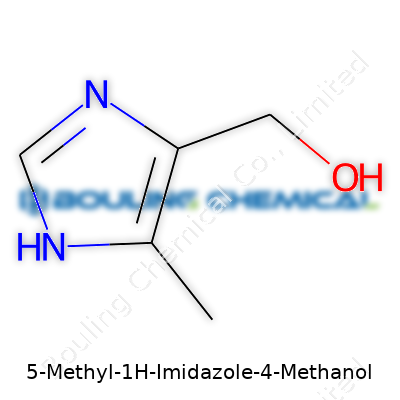
5-Methyl-1H-imidazole-4-methanol, known for its straightforward core structure, includes a methyl group at the 5-position and a hydroxymethyl at the 4-position on the imidazole ring. Researchers turn to this compound as an intermediate when developing potential drugs or specialty agrochemicals. Companies often offer it as an off-white to pale yellow crystalline powder, and chemists find that its dual functional groups support derivatization with a wide range of acyl, alkyl, or aromatic substituents. The molecule’s balance between hydrophilicity and modest hydrophobicity increases its appeal for pharmaceutical design, especially in the pursuit of water-soluble drug candidates.
A lot goes on in a small molecule like this. Under the microscope, 5-Methyl-1H-imidazole-4-methanol usually appears as a discreet crystalline material. Solubility in water and a range of polar solvents lets researchers handle it easily in most laboratory work. Melting points typically fall between 120-130 °C, allowing purification by short-path distillation or simple recrystallization. The imidazole ring supports tautomeric forms, and its methyl group tends to enhance electron density in the ring, changing both acidity and reactivity compared to unsubstituted imidazoles. The hydroxymethyl group offers straightforward chemistry for ether, ester, or oxidized derivatives, which keeps the synthetic possibilities wide open.
Reputable suppliers frequently quote a purity of at least 97% as determined by HPLC or NMR. Labels display the batch number, date of manufacture, shelf life, and recommended storage conditions, which usually call for a cool, dry location away from direct sunlight. Spectra for both NMR and IR support identification, with the methyl singlet near 2.3 ppm in proton NMR and sharp OH/CH stretches in the IR around 3200–3500 cm-1. Documentation for shipments typically includes COA (Certificate of Analysis), MSDS (Material Safety Data Sheet), and detailed synthetic route or trace impurity data for regulated applications.
A common approach starts from 4-methylimidazole, which undergoes formaldehyde condensation to introduce the hydroxymethyl group at C-4. Acid catalysis simplifies the process, often using aqueous formaldehyde and gentle heating. Other methods bring in protecting groups or alternative methylation steps, but the classic condensation wins out for both yield and reliability. Some newer routes employ phase-transfer catalysis, green chemistry aqueous workups, or even flow synthesis to reduce unwanted by-products and cut down on waste streams. Many researchers choose to skip laborious isolation steps by directly transforming crude reaction mixtures into downstream products, banking on high conversion efficiency and straightforward work-up protocols.
The imidazole ring serves as an inviting scaffold for synthetic modulation. The hydroxymethyl group undergoes oxidation to produce the corresponding aldehyde or carboxylic acid. Etherification, acylation, or sulfonation stretch the chemical utility further. The methyl group remains fairly unreactive under normal conditions, but strong oxidants can convert it to aldehydes or acids, enabling preparation of a host of functionalized derivatives. In the presence of nucleophiles or electrophiles, the imidazole nucleus acts as both an acceptor and donor, which explains its frequent selection in the design of ligands, catalysts, or pro-drug forms. Bioconjugation or polymer attachment often use the methanol group as a handle, broadening the application base from chemistry into material science and biomedicine.
5-Methyl-1H-imidazole-4-methanol turns up in catalogs under several variants. Some refer to it as 5-methylimidazole-4-methanol, others as 4-(hydroxymethyl)-5-methylimidazole. In pharmacological discussions, short-hand names like MIM-4-MeOH or just MIMOL take hold. It pays to cross-check registry numbers and detailed molecular structures, since too many imidazole variants exist for casual guesswork.
Even molecules with benign reputations deserve respect. Standard laboratory PPE—gloves, goggles, and fume hoods—applies to 5-methyl-1H-imidazole-4-methanol. Its moderate water solubility promises ease of handling, but skin or eye contact can still cause irritation. Storage in airtight containers keeps moisture and oxygen exposure to a minimum. Waste streams fall under standard organic disposal protocols, though local regulations sometimes call for precaution due to the imidazole core’s slow breakdown in the environment. Operational standards align with ISO guidelines for small-molecule research chemicals, and any product designed for pharmaceutical or food testing must undergo rigorous batch-level analysis.
Demand comes from several sectors. Drug designers value the molecule as a precursor to anti-infective agents, antifungal scaffolds, or enzyme inhibitors. Agrochemists investigate it as a seed treatment additive or as a platform for plant growth modulators. Biochemists sometimes use its derivatives as pH indicators at specific ranges or as buffer additives. More specialized applications touch on polymer chemistry, where the molecule confers basicity or chelation to backbone structures. One may find it in specialty dyes or optical brighteners, thanks to the conjugated imidazole ring adjusting photostability and emission spectra.
Interest in this molecule continues to grow in both academic and industrial labs. Research funding increasingly turns to modification of imidazole structures for resistance management in pathogens or as ways to reduce off-target toxicity in drug candidates. Structure-activity relationship (SAR) studies focus on the influence of its methyl and hydroxymethyl groups on biological targets. Companies with in-house libraries often keep 5-methyl-1H-imidazole-4-methanol at hand for parallel synthesis, seeking new hits in lead optimization campaigns. I’ve seen university groups leverage this molecule in both enzyme mimicry and as reference compounds for complex analytical calibrations.
Two major fronts define toxicity research with 5-methyl-1H-imidazole-4-methanol: acute exposure and long-term effect studies. Data so far suggest relatively low acute toxicity in standard animal models, but higher concentrations provoke irritation and, with chronic exposure, there have been hints of mild hepatotoxicity. Researchers track metabolites and breakdown products, since imidazoles sometimes linger in biological tissues. At this stage, full toxicological clearance hasn’t happened for environmental or dietary use, and biocompatibility still gets checked at both preclinical and regulatory levels. Further research into metabolic fate, along with long-term ecological impact assessments, will determine how the molecule fares outside the lab.
5-Methyl-1H-imidazole-4-methanol sits at the intersection of synthetic utility and biological relevance. Demand for imidazole frameworks remains robust, as more drug candidates require tailored solubility and functional handles for selective conjugation. Green chemistry efforts look to streamline production, shrinking waste and boosting atom economy in scale-up processes. Advances in high-throughput screening, catalysis, and computational design promise fresh insight into reactivity and application profiles. From my own vantage point, the blend of well-understood core chemistry and new possibilities keeps the molecule relevant both now and in the years to come, with a steady stream of publications and patents sure to follow.
Anyone who’s spent time in a pharma lab knows that even tiny molecular tweaks can make a world of difference in a drug’s activity. 5-Methyl-1H-Imidazole-4-Methanol holds a lot of value as an intermediate for synthesizing larger and more complex molecules. Medicinal chemists often modify the imidazole ring to adjust solubility, bioavailability, or target binding. The methyl and hydroxymethyl groups let researchers bolt on new chemical arms for specific tasks. In drug research, chemists have tried out imidazole derivatives for everything from antifungal agents to enzyme inhibitors. Think of this compound as a versatile connector, letting teams try out new drug designs efficiently. According to recent peer-reviewed studies, this class of compounds can enhance antimicrobial properties and help in tuning the pharmacokinetic profile of potential drug candidates.
Modern agriculture depends heavily on innovative chemistry to grow more food with less environmental harm. 5-Methyl-1H-Imidazole-4-Methanol shows up in the synthesis of agrochemicals such as fungicides and herbicides. Its structure makes it useful for building molecules that disrupt harmful microbes or weeds but leave crops unharmed. Over time, the search for more effective pesticides has pushed companies to look for molecules that degrade quickly and don’t linger in soil or water—characteristics that imidazole-based compounds can help deliver. Several patents filed over the last decade point to derivatives of this compound used as building blocks for new crop protection formulas, most notably in wheat and rice cultivation.
Specialty chemicals make their way into everything from industrial coatings to dyes. In many formulations, 5-Methyl-1H-Imidazole-4-Methanol provides a rigid backbone that stands up well under heat or acidic conditions. This means less degradation and longer product life in things like water-resistant coatings or specialty inks. Chemists value the stability and reactivity balance that the imidazole core brings, allowing them to design polymers with better performance than older alternatives. From my time working with polymer scientists, I’ve seen firsthand how small changes in a molecule’s side chain can lead to products that last longer in harsh environments, such as outdoor signage or marine equipment. Industry reports back this up, showing a steady increase of imidazole-based compounds in protective materials and high-performance resins.
Researchers searching for sharper diagnostic tools often turn to molecules like 5-Methyl-1H-Imidazole-4-Methanol. This compound serves as a starting point for linkers and tags in biochemical assays. Its functional groups attach easily to various fluorescent dyes or affinity labels, improving sensitivity when measuring the presence of specific proteins or DNA in a sample. In the age of precision medicine, this matters: rapid, accurate tests drive better outcomes for patients. Academic articles from the last five years show a clear uptick in custom reagents built around imidazole scaffolds, whether for laboratory diagnostics or emerging point-of-care technologies.
Every time a new chemical comes on the market, environmental safety jumps to the front of the list. Regulators and industry leaders ask tough questions about toxicity, residue, and breakdown products. For 5-Methyl-1H-Imidazole-4-Methanol, several independent labs have started assessing its behavior in water and soil. Early results suggest manageable breakdown and low persistence with proper use—a relief for environmental chemists and regulatory teams. Manufacturers still have to fine-tune production to prevent impurities, and researchers continue measuring exposure risks, especially for people handling concentrated forms of the compound.
Teams working with 5-Methyl-1H-Imidazole-4-Methanol keep chasing safer, greener syntheses. Green chemistry approaches, such as solvent-free reactions or enzymatic methods, could cut down on waste and energy use. On the pharmaceutical side, more collaboration between industry and academia may open new uses for this versatile building block, with a focus on personalized medicine. Drawing from past experience developing specialty materials, I see a lot of room for scientists to engineer even more targeted, less polluting uses for this compound in the future.
Chemists talk a lot about purity for a reason. Dealing with something like 5-Methyl-1H-Imidazole-4-Methanol, the actual percentage of pure compound in a bottle makes a real-world difference. Synthetic reactions turn out one way using a 98% pure sample, something else if that drops to 90%. Impurities, even dust-sized, can throw off yields, cause side reactions, and complicate results. High-purity material matters most where trace side products create confusion—analytical labs, pharmaceutical research, material sciences. Grades range from “technical” for industrial use to “reagent grade” or “analytical grade” where a number over 98% signals confidence that tests will show what they’re supposed to show.
Most sources define chemical purity for 5-Methyl-1H-Imidazole-4-Methanol over 97% for research grade, with certificates of analysis listing any other compounds in that last few percent. These technical details sound small but carry weight. Chromatographic methods—gas or liquid—separate out every molecule mixed in. Something labeled 99% pure might still carry one percent of unknowns, putting a wrench in a long reaction chain. In drug chemistry, that trace amount gets scrutinized through extra steps—NMR, HPLC, elemental analysis. One odd peak crops up and weeks of effort sometimes go wasted.
Buying chemicals always turned into a process. A batch with no paperwork raised alarms, even for something cheap or routine like imidazole derivatives. Strong labs want a certificate of analysis showing the exact content—the melting point, NMR spectra, water content, even heavy metal traces. Not every supplier gives this willingly, especially with rarer compounds. If the paperwork just gives a flat “>98%,” further independent testing becomes necessary if results need backup.
The industry uses short labels—reagent, analytical, USP, technical. High school practical classes can get by on technical grade for cost savings. Pharmaceutical or biotech firms, in contrast, expect nothing less than confirmed 99%+ with fewer than 0.1% heavy metals or specific toxins. Confidence builds on transparency. Every step in creating a drug or testing a new catalyst rests on knowing exactly what went into the flask.
Trace contamination sneaks past one supplier’s standards more times than most admit. Better regulation from both government and industry groups leads to more reliable chemicals on the market. I keep pushing for clearer labels—saying not just “high purity” but backing it with real data, open to scrutiny. Encouraging more open sharing of analysis between labs, even informally, means that those working outside major institutions don’t feel left behind.
A lot of chemists would welcome smart tools for instant verification—portable spectrometers, cloud-shared analysis results—so purity checks don’t stop with a sheet of paper. For 5-Methyl-1H-Imidazole-4-Methanol, just knowing the exact number, down to the decimal, turns risk into reliability. The job calls for honesty, detail, and a willingness among suppliers to face tough questions. That’s what keeps experiments meaningful and results alive.
Too many folks shrug off lab chemicals as just another item on the stockroom shelf. In my years around research benches, I’ve learned each compound comes with its own quirks, and 5-Methyl-1H-Imidazole-4-Methanol stands out as one that rewards respect. With its structure, you get a compound that brings moisture sensitivity and a degree of volatility—a duo that doesn’t forgive laid-back storage habits. Whether it lands in an academic lab, industrial setup, or a small startup, sloppy handling can spark trouble for your team and research.
The smart move begins by reading up on reliable sources—manufacturer labels, current material safety data sheets, and peer-reviewed chemistry references. I’ve seen too many accidents caused by someone trusting a scrap note or a dusty old binder instead of recent data. This compound thrives in a cool, dry spot, far from heat sources or direct sunlight. I put my trust in flammables cabinets if they’re available, even if a chemist swears something isn’t as “volatile as acetone or ether.” Humidity eats at this molecule’s shelf life, so a desiccator or a tightly sealed bottle delivers real benefits.
Contact with other reactive materials spells risk. I’ve worked in places where bulk alcohols lived side by side with oxidizers. One careless spill or mix-up leads to a mess. Segregation matters. Put imidazoles apart from acids, peroxides, and oxidizing agents. A metal shelf that’s free from rust and residue cuts down on surprise reactions.
Routine breeds accidents. In my experience, even veteran chemists drop their guard if the bottle looks familiar. Every transfer, weighing, or sample draw gets nitrile gloves, protective eyewear, and a no-nonsense lab coat. Splash goggles beat glasses—chemical burns aren’t a rite of passage. Good air circulation wins out over bravado; use the fume hood any time the cap comes off, since vapors can contribute to breathing trouble over time.
Let’s talk clean-up. Spilling a few milligrams feels minor, yet surfaces and tools pick up residues quick. Grab those compatible spill pads, not paper towels that spread the mess. Dispose of soiled gloves and absorbents in proper containers, never the trash under your desk. Waste collection should line up with established chemical hygiene plans; surprise inspections have upended labs that treated waste as an afterthought.
A solid inventory saves headaches. Know how long each bottle has been on the shelf, and don’t hesitate to label dates of opening. Outdated chemicals bring uncertainty and have caused ruined reactions in my hands more than once. Training matters as much as physical controls. If only one person in the lab knows the drill, the rest fall back on guesswork during a spill or emergency.
Regulation compliance isn’t paperwork for its own sake. Regulatory violations often start with small shortcuts—a missed label, ignored incompatibility, or propped-open storage door. Regular audits beat spreadsheets that never see the light of day.
A careful lab culture grows from the bottom up. The lessons above may sound basic, but they prevent accidents and preserve resources. Whether you’re finishing a thesis or running a production floor, storing and handling 5-Methyl-1H-Imidazole-4-Methanol calls for awareness, up-to-date knowledge, and respect for both health and results. Mistakes leave marks on projects, careers, and sometimes lives. Smart storage and handling never cost more than learning by mishap.
My own experience with organic chemicals like 5-Methyl-1H-Imidazole-4-Methanol taught me that not all hazards hit you in the face with a strong smell or obvious warning sign. This compound, common in research spaces and specialty manufacturing, carries health risks that can't be ignored. Toxicity data from sources like the ECHA emphasizes its potential to irritate the skin, eyes, and respiratory tract. Long-term exposure hasn’t been studied as thoroughly as some mainstream industrial solvents, so working with it demands extra vigilance.
Holding a bottle of this imidazole derivative in the lab, I always checked for fully intact gloves. Nitrile or butyl rubber gives the best protection against permeation. Thin latex tears easily, especially during repetitive tasks. Even a brief splash on unprotected skin left a dry, red mark, confirming the Material Safety Data Sheet’s guidance. Splashing into eyes burns—goggles or a face shield matter far more than you’d guess before you’ve felt it yourself.
Working with potentially volatile or irritating chemicals, I also learned that proper ventilation changes everything. Labs without local exhaust or fume hoods quickly fill with vapors, especially during weighing and dilutions. Fume hoods trap vapors right where they form, making open benches a bad spot to handle the powder or its solutions.
Chemicals like 5-Methyl-1H-Imidazole-4-Methanol belong in tightly sealed containers, away from strong oxidizers and moisture. I once watched a mislabeled bottle left open overnight, only to find the entire outer surface caked with crystals the next day. Moisture and air react with imidazoles. They degrade, forming unknown by-products and raising contamination risks. Using amber bottles keeps the light out—many imidazoles break down faster under harsh lighting, and that ruins product purity. Shelf life drops off fast in the presence of any heat source, so a cool storage spot matters.
Labs sometimes skip fire safety with compounds that don’t ignite easily, yet accidents happen where you least expect them. 5-Methyl-1H-Imidazole-4-Methanol does not light up like ethanol, but it burns if given enough heat. I saw a colleague knock a hot plate into a spill—the result was acrid smoke and a hasty evacuation. Sand, not just lab wipes, put out the small flames. Fire blankets and extinguishers should stay close by. Never forget the importance of well-marked, unblocked exits.
Spills feel stressful, but calm cleanup makes all the difference. Workplaces using this chemical store absorbent pads and bags labeled for hazardous chemical waste. A hazardous waste bin stands within arm’s reach in most research labs. Immediate cleanup with gloves and goggles on, followed by wiping the space with mild detergent, removes residue and keeps surfaces safe for the next experiment.
None of this matters without regular training. OSHA guidelines push for annual refreshers, not just for newbies. Everyone gets rusty, me included. Every time I trained someone new, walking them through the safety sheets took on new meaning. Documenting exposures, even minor ones, helps spot patterns and shape future protocols. A clear record of incidents or close calls means better planning next time and keeps everyone safer.
The lessons learned carry over between all kinds of chemicals. 5-Methyl-1H-Imidazole-4-Methanol shows why planning and careful work habits protect not only health but also scientific results and equipment. Good routines, up-to-date resources, and team awareness make the difference between smooth work and preventable danger.
Chemical supply rarely makes headlines until someone needs a specialty molecule and finds it tough to track down in large amounts. 5-Methyl-1H-Imidazole-4-Methanol fits that niche category that most of us outside of advanced chemistry circles haven’t heard about. Dig a little, and you start to understand its role in research labs, pharmaceutical development, and possibly for new types of industrial catalysts.
Researchers and product engineers have good reason to care about sourcing simplicity. Running experiments at bench scale is one thing; ramping up to pilot or production batches demands steady access and consistent purity. With 5-Methyl-1H-Imidazole-4-Methanol, labs often request only a few grams at a time. Once a project gets legs, the search for kilograms often starts in earnest. Several online distributors show this compound in their catalog, but not every supplier can actually back up claims of large-volume fulfillment. Requests for 10, 25, or 100 kilos prompt the real indicator—how established a global supply chain has become for that compound.
Bulk stock depends on both demand and safety knack. Specialty chemicals like this don’t get the same production scale as basic commodities such as ethanol or acetone. Most chemical plants won’t keep thousands of kilos of 5-Methyl-1H-Imidazole-4-Methanol just waiting for a taker. If my own experience calling up suppliers teaches anything, smaller distributors may need to source from their own upstream partners, sometimes delaying delivery by weeks or more.
For pharmaceutical researchers, uncertainty over bulk access can slow everything. Take a small company developing a drug that requires this compound as an intermediate. They can prove the concept in tiny batches and then hit a wall—their project budget won’t allow custom synthesis on every order. Prices climb fast once suppliers move away from stock and toward “custom by request.” Add in international shipping controls, import/export paperwork, or even basic hazards associated with specialized compounds, and routine purchasing becomes an ongoing battle.
In chemistry, one batch does not guarantee the next. Variations in synthesis routes, sources of starting material, or even shifting regulatory requirements can trip up buyers. Broad claims about available stock don’t always align with actual ability to deliver a certificate of analysis or a consistent product across production runs. I’ve seen labs jump through hoops retesting batches sent air-freight from new sources to keep vital work on track.
Finding reliable suppliers becomes less about the search for lowest price and more about building trust. Buyers call established producers, ask about batch sizes, and require detailed safety documentation. This mirrors my own experience: relationships matter as much as technical spec sheets—especially with less common chemicals.
Collaboration between chemical producers and research buyers drives real progress. Upstream transparency about availability and lead times helps scientists plan better. More producers could open those channels, sharing clear info about batch size limits and realistic timelines, to help customers avoid order snags. Financing advance purchase orders or pooling bulk needs among several labs might further ease access, smoothing out both price and supply.
The search for 5-Methyl-1H-Imidazole-4-Methanol in bulk brings up those perennial lessons in applied chemistry—blending patience, persistence, and networking is often as important as anything done inside the lab.
| Names | |
| Preferred IUPAC name | (5-methyl-1H-imidazol-4-yl)methanol |
| Other names |
4-(Hydroxymethyl)-5-methylimidazole Imidazole-4-methanol, 5-methyl- 5-Methyl-4-imidazolemethanol |
| Pronunciation | /ˈfaɪˈmɛθɪl waˈneɪtʃ ənˈɪmɪdəˌzoʊl fəˈmɛθənɒl/ |
| Identifiers | |
| CAS Number | [731772-34-6] |
| 3D model (JSmol) | `3D Model (JSmol) string` for **5-Methyl-1H-Imidazole-4-Methanol**: ``` CC1=C(N=CN1)CO ``` *(This is the SMILES string, which is used by JSmol to render the 3D model.)* |
| Beilstein Reference | 1340792 |
| ChEBI | CHEBI:16706 |
| ChEMBL | CHEMBL502271 |
| ChemSpider | 14640333 |
| DrugBank | DB08798 |
| ECHA InfoCard | 100.153.441 |
| Gmelin Reference | 87469 |
| KEGG | C06348 |
| MeSH | D000068345 |
| PubChem CID | 11692716 |
| RTECS number | UJ8923000 |
| UNII | 4EA8306T2F |
| UN number | Not regulated |
| Properties | |
| Chemical formula | C5H8N2O |
| Molar mass | 98.12 g/mol |
| Appearance | White to light yellow solid |
| Odor | characteristic |
| Density | 1.24 g/cm³ |
| Solubility in water | slightly soluble |
| log P | -0.22 |
| Vapor pressure | 7.5E-4 mmHg at 25°C |
| Acidity (pKa) | 12.04 |
| Basicity (pKb) | 7.39 |
| Magnetic susceptibility (χ) | -72.2×10^-6 cm³/mol |
| Refractive index (nD) | 1.547 |
| Dipole moment | 2.99 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 122.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -23.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3895 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P321, P362+P364, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | > 174.9 °C |
| Lethal dose or concentration | LD50 oral rat 1000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2200 mg/kg (rat, oral) |
| NIOSH | Not established |
| REL (Recommended) | 0.05 ppm |
| Related compounds | |
| Related compounds |
1H-Imidazole-4-methanol 5-Methylimidazole 1-Methylimidazole Histamine 4(5)-Methylimidazole Imidazole |