Few chemicals in the world come with a layered backstory like 5-Ethylphenazine. Post-war chemistry kicked off an arms race in dye synthesis and antimicrobial discovery. Phenazine compounds surfaced as interest grew around bacterial pigments with suppressive powers. 5-Ethylphenazine did not pop up out of nowhere. Generations of lab notebooks tell a story of test tubes lined up by the hundreds, attempting to tweak the basic phenazine skeleton with different side chains. Adding an ethyl group at the 5-position gave researchers a new angle to probe. Decades ago, this tiny twist provided just enough difference to spark studies in both coloration and bacterial inhibition, especially as the medical field sought better disinfectants in the face of emerging resistance. Over time, production methods improved, shifting from low-yield, small-batch syntheses to more scalable approaches. With every decade, interest flared up again, as its uses in analytical chemistry and biological research expanded.
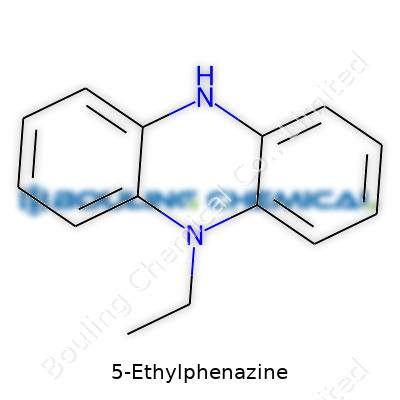
Walk into any advanced chemical supply house and chances are, someone has 5-Ethylphenazine on the shelf. Chemists who know their stuff remember it as a sharp, crystalline powder, yellow to reddish in color, drawing eyes away from the bland monotony of most fine chemicals. While not as famous as antibiotics or bulk reagents, it holds a place of respect among researchers picking apart oxidative processes or testing new routes in organic synthesis. Trade catalogs can cause headaches with minor variants, but the true product always centers on the phenazine core—two benzene rings fused around two nitrogen atoms—anchored firmly by that ethyl tail at position 5.
People who’ve handled a beaker of 5-Ethylphenazine know it’s far from the typical white powder crowd. With a melting point in the range of 150-160°C and a strong, distinctive color, it gives rapid clues about its structure to the practiced eye. The ethyl group adds a touch of hydrophobicity, which can shift its solubility just enough to separate it from plain phenazine. This brings practical advantages, since not every experiment wants a compound that dissolves with nothing but a suspicious glance from a flask of water. Its stability at room temperature has made it reliable for storage and inclusion in composite chemical kits. The molecule resists casual oxidation and photodegradation, offering a healthy shelf life in standard, dry conditions.
There’s no mystery to quality when it comes to purchasing this compound for research or manufacturing. Reputable suppliers print the molecular formula (C14H12N2), list the CAS number (3101-39-9), and display purity—a standard minimum of 97% for most analytical work. Safety labels deliver the universal pictograms for irritants and hazards. Clear storage guidelines—store in a cool, dry place, and avoid excessive light—appear on every container. Those specifics make a chemist’s job easier, setting reliable expectations about what’s going into a reaction or assay.
Lab stories about 5-Ethylphenazine most often start with a careful build-up from smaller aromatic molecules. Synthesis springs from the condensation of o-phenylenediamine with ethyl-substituted quinones. Catalysts like oxidizing acids keep the reactions humming, pushing electrons around to knit together those carbon-nitrogen bonds. Older protocols required painstaking separation and purification, with repeated recrystallization to chase off byproducts. Modern tweaks have given better yields by streamlining the solvent and temperature controls. Some chemical engineers have even found ways to adjust the electronics around the phenazine ring to tilt the synthesis in favor of the ethyl variant, reducing waste and cost in the process.
Chemically, 5-Ethylphenazine acts as both a participant and a tool. Its nitrogens stand ready to be protonated under acid, generating vivid salts. Electrochemical studies use this molecule to shuttle electrons by cycling between oxidized and reduced forms, a trick that’s especially useful when probing cellular respiration analogs. Substitution at the ethyl or even the ring positions sometimes leads to new derivatives—some more water-hungry, others more fat-soluble—with each change unlocking unique color shifts or biological activities. These tweaks matter since drug discovery and dye development both thrive on the gentle push and pull between hydrophilic and hydrophobic domains. Adding halogens, lengthening the side chain, or introducing amino groups has led to many a paper and patent over the years.
The world of chemical names spins its own web. Alongside ‘5-Ethylphenazine,’ catalogs or papers toss around tags like ‘1,6-Diazaphenanthrene, 5-ethyl-.’ European suppliers have used ‘NSC 109193’ and similar codes over the years. Sometimes the simple ‘5-ethyl-phenazine’ pops up. Despite the confusion, the core structure always remains. Practically, catalog numbers and CAS identifiers have become lifesavers in ordering and lab inventory, especially as research collaborates across borders.
Safety with phenazine derivatives means more than just gloves and goggles. Vapors rarely bother the nose, but dust or contact can irritate skin and eyes. Inhalation creates risk if fine powders go airborne. Most workplaces enforce fume hoods and sealed containers for weighing, minimizing exposure. Disposal rules reflect its persistence—waste streams get segregated and sent for specialized treatment. Safety Data Sheets provide real-world warnings rooted in decades of cautious handling, especially since the molecule resists easy breakdown. Long-term storage calls for glass or inert plastic, away from acids or oxidizers, to ensure stability and lab safety.
Research dominates the demand for 5-Ethylphenazine. Labs studying bacterial electron transport love its redox power and color signals. Microbiologists probe its use as a selective agent or bacterial growth marker, taking advantage of how it can both signal and interfere with respiration. Biochemical screens have used its derivatives to test for new antibiotics or to trace the rise and fall of oxidative stress. The dye industry once considered its colorfastness and hue, although larger-scale pigment production settled mostly on more cost-effective compounds. In analytical chemistry, its predictable behavior has inspired assays for metal ions and organic pollutants.
Innovators keep circling back to 5-Ethylphenazine for more than nostalgia. Interest in antibiotic resistance has put phenazine structures under the microscope—literally. Medicinal chemistry teams synthesize analogs, seeking better wound treatments or disinfectants. On the electrochemical front, researchers have fitted the molecule into polymer films and nanomaterials for sensors. Computational chemistry models have grown sharper, helping spot subtle tweaks that might improve bioavailability or reduce toxicity. The drive to move from flask synthesis to greener production processes has also grown, with efforts directed at water-based reactions and recyclable catalysts.
No chemical with biological activity escapes toxicologists for long. 5-Ethylphenazine earns scrutiny for its mild irritant effects in contact studies and its potential impacts when introduced to living tissue. Animal research points to relatively low acute toxicity for one-off exposures, but chronic effects—especially on the liver and kidneys—get regular study updates. Water system monitoring keeps an eye on its persistence, since phenazines resist breakdown in municipal treatment. Its derivatives sometimes pop up in antimicrobial screens, raising eyebrows about long-term ecological effects. Regulatory agencies recommend tightly controlled use, and they call for more ecological studies as research broadens.
The story of 5-Ethylphenazine hardly feels finished. Researchers continue to invent with this structure in mind, especially as pressure grows to find new antibiotics and sustainable dyes. Advances in green chemistry hint at cleaner, safer production methods on the horizon. New spectroscopic tools may unlock even more subtle applications as redox agents in both medicine and material science. Toxicity research points the way to safer handling and better disposal, especially as environmental regulators tighten standards. In the end, its blend of storied history and modern relevance keeps 5-Ethylphenazine a peculiar but persistent presence—proof that chemistry never truly throws away a working molecule.
Most folks haven’t heard of 5-Ethylphenazine unless their daily work involves pipettes or fermentation tanks. This molecule doesn’t show up on ingredient lists in snack foods or pop up during routine household chores. That’s probably a good thing. The world of synthetic chemistry and microbial biotechnology gives us a laundry list of complex names, but every so often, one of those compounds manages to punch above its weight. With 5-Ethylphenazine, researchers see more than just a lab curiosity.
The spotlight often turns to antibiotics or antiseptics when the conversation lands on keeping infections at bay. Still, many of the drugs we rely on are losing their punch. In my own run-ins with the healthcare system, I’ve watched doctors scramble for effective tools as bacteria change up their defenses. Here, 5-Ethylphenazine steps in with an interesting track record.
Scientists discovered 5-Ethylphenazine as a byproduct from some strains of Pseudomonas, a wily breed of bacteria that usually pops up in soil and water. Instead of being a threat, this chemical acts in the opposite direction: it hurts other microbes by disrupting their ability to generate energy. That could mean new options for treating tough infections, which feels urgent in a time when superbugs leave whole hospital wards on edge.
It’s easy to leave 5-Ethylphenazine tucked away in published studies, but its uses look much broader. Researchers consider whether this compound could help control plant diseases in agriculture. Anyone who has tried to grow tomatoes or potatoes in the backyard knows the frustration when blights spread fast and farmers often cycle through chemical treatments. By introducing naturally derived substances like this one, we might see lower reliance on aggressive synthetics, which can linger in the soil and water.
Factories also see some promise. Chemical companies keep searching for catalysts that push reactions faster without needing a battery of expensive metals or harsh conditions. 5-Ethylphenazine’s structure interacts with electrons in just the right way to mediate biochemical reactions. For example, certain dyes and electronic materials benefit from these reactions, leading to more stable products or smaller waste streams. Every time I see a warning sign near a chemical plant or dye factory, I think of ways to ease up on the environmental toll. Compounds that get the job done cleanly sit high on wish lists.
5-Ethylphenazine doesn’t solve every problem, but ignoring it would mean missing out on new directions in healthcare, farming, or even electronics. If drug designers dig deeper, we might find antibiotics that turn out better than what’s on shelves now. Farmers could add this compound to a roster of eco-friendly tools to keep crops healthy. Industry leaders focused on cleaner chemistry might soon have another arrow in their quiver.
Of course, the fine print matters. Every new substance raises questions about safety — for humans, animals, and ecosystems. In my experience, breakthroughs land best when they arrive alongside tough, open conversations about risks and responsibility. Given its background and current interest, 5-Ethylphenazine is more than a tongue-twister in journals. It’s a story working its way from test tube to real life, and the chapters ahead look worth following.
Chemistry gets a kick out of names that sound complex. Take 5-Ethylphenazine, for instance. That’s a mouthful, but you break it down, and it’s really just a mash-up of phenazine with an extra ethyl group hanging off the fifth spot. Most folks have never seen a phenazine in their day-to-day lives, but you’d be surprised how often related compounds pop up across industries—from colorants to antibiotics.
The backbone comes from phenazine, a fused aromatic ring that looks like two benzene rings sharing a pair of nitrogen atoms. Phenazine’s core feels stable, thanks to its flat, aromatic system. Chemists like this structure because it carries electrons neatly around the rings, which helps in all sorts of redox reactions. You toss an ethyl group (–C2H5) onto the fifth carbon on one of those rings. That’s how 5-Ethylphenazine stands out from regular phenazine. The extra two-carbon chain doesn’t just sit there; it changes how the molecule interacts with other chemicals, and sometimes, with living systems.
Back in the lab, I’ve seen structures like this throw off different behaviors based on small tweaks, especially when you fiddle with the placement of hanging groups. Slide that ethyl group around, and you often get a whole new set of properties. The fifth position isn’t an accident—it matters because the way the electrons swirl around those rings, the fifth spot gives the new group extra influence on how the molecule interacts with light, heat, and biological systems.
This specificity can create potent dyes or powerful antibiotics. Pseudomonas bacteria, for instance, churn out phenazine derivatives that go to work in their microscopic turf wars. Change just one group on the ring, and suddenly you have an agent that disrupts other microbes or carries electrons in fuel cells. So each tweak—like the ethyl group in 5-Ethylphenazine—unlocks a new set of tricks.
If you had a piece of paper and tried to draw 5-Ethylphenazine, you’d start with two hexagons joined together, each representing a benzene ring. Slide in two nitrogen atoms at the first and fourth positions on the ring system. At carbon number five, count it out, and attach that ethyl group. It’s not just doodling—sketching molecules gets you thinking about how each atom pulls and tugs on its neighbors. In practice, this small detail can decide everything from color to chemical reactivity.
The real trick with chemicals like 5-Ethylphenazine is giving them purpose. It’s one thing to map out a structure, another thing to turn that structure into something useful. Chemists don’t stop at theory—they take these skeletons and test them for properties like antimicrobial activity or as candidates for electronic materials. Sometimes, you find that one small group like ethyl makes the difference between a dud and a blockbuster. This highlights the need for ongoing exploration, not just in labs, but also in the industries that pick up these discoveries and run with them.
Looking at 5-Ethylphenazine, the lesson is clear: little tweaks in chemical structure can open the door to big changes in function. Whether you’re in a lab or working in industry, you’re always just one bold substitution away from an unexpected breakthrough.
Everyday life comes with chemical buzzwords. Some stick in our minds because we hear them at work, in science news, or even in product recalls. 5-Ethylphenazine doesn’t exactly sound like a household name, but stories about hazardous chemicals never seem too far away. So let’s talk about this one: what kind of risk does 5-Ethylphenazine bring to the table?
Back in college, I spent time around chemistry labs and got used to the endless parade of glass flasks stuffed with colorful liquids. We always took extra care with anything marked “phenazine,” and 5-Ethylphenazine turns out to be just one variation among dozens in that family. It's got uses that pop up in specialized research, sometimes in industry, and even in rare biochemistry experiments.
No matter where it’s found, the same rule always comes up: respect the material safety data sheet. This one flags up some typical risks. It irritates the skin and eyes. Inhalation isn’t a good idea for obvious reasons—breathing in chemical dust never helps lung health. If you swallow it, expect stomach pain or nausea. Signs point to this chemical being no one’s idea of a safe ingredient list.
There’s a bit of a myth that only famously deadly chemicals create real trouble. 5-Ethylphenazine stands as a reminder that less headline-grabbing stuff still deserves concern. Hazards might not mean instant danger at low doses or with normal use, but that doesn’t free users from real risk in certain settings. I’ve seen people get careless with supposedly “routine” compounds and wind up in the health clinic—always a wake-up call.
The concern isn’t only about accidents. Repeated contact with compounds like these sometimes leads to cumulative problems. You wash your hands, but some residue stays behind; you touch your face or your lunch; problems can sneak up regardless of the process you follow. Some studies have shown compounds in the phenazine family cause cellular damage with repeated exposure, especially in laboratory animals.
So what pushes things in the right direction? Common sense has always meant more than just reading the label. Any lab or plant handling specialty chemicals benefits from sharpened routine—good ventilation, protective gear, and storing chemicals out of reach when not in use. At my first research job, the lab manager never let us get away with shortcuts. Simple habits made all the difference; gloves, goggles, and the habit of never eating or drinking around chemicals stopped a lot of avoidable mistakes.
Regular risk assessments help too—don’t assume last year’s practices still fit if you switch suppliers or ramp up production. Anyone who supervises staff processes needs refresher training more often than industry regulations might suggest. Strong protocols also control chemical inventory, so nothing sits forgotten at the back of a shelf, quietly breaking down or leaking.
One big hazard shows up after use: improper disposal. Pouring unused chemicals down the drain, for example, pushes trouble toward city wastewater treatment. Municipal systems rarely scrub out specialty organics like 5-Ethylphenazine. Contaminated water goes right back out to rivers, affecting aquatic life and, eventually, human water supplies. Hazardous waste disposal costs time and money but ignoring this step makes the risk ripple into new areas.
Everyone who crosses paths with 5-Ethylphenazine can take steps to minimize risk. Treat it the same way you’d treat an unfamiliar cleaning chemical or medication: respect the warning labels and play it safe. The more often we see stories of casual handling leading to accidents, the more it encourages a culture of caution. That’s the quiet, smart side of dealing with hazardous chemicals—never flashy, always important.
5-Ethylphenazine belongs to a group of organic compounds with a reputation for both usefulness and danger. Even if it only sounds like a term reserved for a lab manual, this material finds a place in research, sometimes appearing in chemical manufacturing. Users tend to get comfortable with familiar substances, and that leads to trouble. Earlier in my career, I saw a colleague shrug off a chemical’s storage label. A week later, his carelessness forced the team to evacuate a floor of the building. Sometimes learning the hard way leaves a message that sticks: these chemicals carry weight.
With 5-Ethylphenazine, the risks tie straight back to its chemical structure. Many phenazines show some instability, especially under light, heat, or air exposure. That means left open on a shelf, or stored next to flammable liquids, a bottle can turn from boring to hazardous with little warning. Fire risk, inhalation toxicity, skin sensitivity—these hang over each container. At the end of the day, proper storage means people walk back home instead of heading to the hospital.
The safest spot for 5-Ethylphenazine is a cold, dark, and well-ventilated cabinet, away from sparks, open flames, and hot equipment. I always look for a cabinet certified for storing flammables, fitted with sturdy shelving, and grounded to avoid static buildup. I’ve worked in labs that cut corners, storing reactive chemicals next to acids or oxidizers. That mix leads to chemical reactions that could have been avoided with a little organization.
Sealing containers tightly protects from both accidental spills and vapor release. Once, during a hot summer, a poorly closed bottle built up pressure and popped open overnight. The clean-up took hours, but the lesson stuck with me: check, and double-check, every seal. Any label with fading ink gets replaced right away. Legibility is just as critical as the right temperature or location.
Regulatory laws exist for a reason. The Occupational Safety and Health Administration (OSHA) and local fire codes often lay out expectations about volatile organic material. Every lab manager shoulders the responsibility for making sure those rules stay front and center. Even small amounts of non-compliance—like stacking too many bottles together, or letting inventory get out of date—can result in fines, lost licenses, or worse, injuries.
Anyone working with 5-Ethylphenazine should have personal protective equipment (PPE) close at hand: gloves that resist punctures, goggles, and lab coats. Ventilation matters—simple fans don’t cut it. Mechanical fume hoods or specialty exhaust systems are the right tools. Emergency spill kits should sit within arm’s reach. Training should never turn into a mindless checklist. Regular drills and refreshers keep the sharp edge on safety habits.
Inventory systems do more than control loss—they track shelf life and flag expired material. Old stocks of chemical linger far too long when no one keeps count, and expired substances raise the risk of surprise reactions.
It’s tempting to view storage as a background concern, something less exciting than the science itself. Yet, overlooking the basics courts disaster. Every safe lab I’ve visited runs on muscle memory: open the cabinet—check conditions—close it right—update the log. These habits protect lives—there’s no simpler or clearer reason to do things right.
A lot of people hear about organic molecules without pausing to consider the real details that go into figuring out characteristics like their molecular weight. On paper, “5-Ethylphenazine” might sound like a mouthful, something tucked deep in a chemistry textbook or mentioned in passing by researchers in white lab coats. But breaking it down helps shine some light. Each atom in its framework counts toward the molecule’s final tally. This number has real consequences—whether you’re mixing up a chemical reaction, planning a synthesis, or just curious about what you’re dealing with in practical lab work.
First, identifying what’s inside 5-Ethylphenazine clears up the road. The core is a phenazine ring—a system most chemists recognize as made up from twenty atoms: two nitrogens and the rest carbons and hydrogens organized in a fused-ring setup. The “5-ethyl” part tells you there’s a two-carbon chain sticking out at the fifth position. Adding everything up, the formula comes out to C14H12N2. From there, a bit of back-of-the-envelope math gives you everything you need for molecular weight:
Add those together and you land on 208.28 g/mol. Notice that every digit counts: making a mistake here leads to headaches later, especially if you scale up any experiment. Mistakes drive up costs, and in some industries, they can put safety at risk.
Plenty of folks outside labs would be surprised how easily a slip in molecular weight messes up calculations in medicine, farming, or food. Digging into this number matters, because it determines how much raw material you weigh on your scale. For researchers in the field, an off-by-one error throws off yields, purity, and dosing calculations that feed into bigger problems, like product recalls or failed experiments.
Back in my university days, we’d prep solutions for analytical chemistry labs, and some students guessed weights for similar molecules, thinking close was good enough. That shortcut cost them precious lab time and ruined hours of careful work. It’s easy to think the details don’t matter until you see the consequences for yourself.
Molecular weight doesn’t live in a vacuum. Every part of industry—whether pharmaceuticals mixing ingredients for a tablet, or companies trying to make dyes—grapples with this number. Quality control teams double check the math. Some companies go as far as to use automated systems, but even those require someone knowledgeable to spot-check for software hiccups. In the research world, the need carries over: drug discovery, environmental monitoring, and even simple teaching labs all rely on accurate values.
One solution: promote hands-on education for students, not just memorizing periodic tables but making them work out calculations every time. Double-checking with reference databases prevents rookie errors, especially in early career researchers. Teams that foster open communication don’t let mistakes linger, since someone is always ready to ask, “Does this number add up?” Care in these steps builds trust in results, not just in chemistry but in the decisions that flow from it.
Details make all the difference in science that affects everyday products. 5-Ethylphenazine, like many molecules, reminds us that accuracy isn’t just a box to tick—it’s the backbone for every discovery, every process, and every product that finds its way from lab to world.
| Names | |
| Preferred IUPAC name | 5-ethyl-2,3-diazaphenazine |
| Other names |
5-Ethyl-1,6-phenazinediamine 5-ethyl-phenazin Phenazine, 5-ethyl- |
| Pronunciation | /ˌfaɪˌɛθ.ɪl.fəˈnæz.iːn/ |
| Identifiers | |
| CAS Number | [2538-52-7] |
| Beilstein Reference | 1639329 |
| ChEBI | CHEBI:72758 |
| ChEMBL | CHEMBL151901 |
| ChemSpider | 21633425 |
| DrugBank | DB13135 |
| ECHA InfoCard | 100.103.957 |
| EC Number | 620-745-9 |
| Gmelin Reference | 87721 |
| KEGG | C18078 |
| MeSH | D010622 |
| PubChem CID | 180799 |
| RTECS number | SE8575000 |
| UNII | 8Q3A4AG3BI |
| UN number | UN3086 |
| Properties | |
| Chemical formula | C14H13N2 |
| Molar mass | 208.27 g/mol |
| Appearance | Red powder. |
| Odor | Odorless |
| Density | 1.146 g/cm3 |
| Solubility in water | Insoluble |
| log P | 2.89 |
| Vapor pressure | 2.64E-4 mmHg at 25°C |
| Acidity (pKa) | 5.63 |
| Basicity (pKb) | 5.95 |
| Magnetic susceptibility (χ) | -63.5 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.661 |
| Viscosity | 3.41 cP |
| Dipole moment | 2.17 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 340.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Harmful in contact with skin. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements for 5-Ethylphenazine: "P261, P280, P305+P351+P338, P337+P313 |
| Flash point | 108°C |
| NIOSH | SN1575000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg |
| Related compounds | |
| Related compounds |
Phenazine 1-Ethylphenazine 2-Ethylphenazine 5-Methylphenazine 5-Propylphenazine |