Chemical innovation rarely follows a straight line. The journey to 5-Ethoxy-2-Mercaptobenzimidazole began in the mid-20th century along with surging interest in benzimidazole derivatives. Back then, chemists were exploring sulfur and nitrogen rings for anti-ulcer, fungistatic, and antioxidant activity. You had the mainstay of early ulcer medicines spring from the benzimidazole backbone. The addition of an ethoxy group—a game-changer—emerged out of persistent tweaking to boost pharmacological traits and solubility, and to steer the molecule’s power in certain applications. My own experience tells me this field has always rewarded curiosity; the willingness to substitute, to shuffle atoms, is what leads to new classes of protective agents and catalysts.
5-Ethoxy-2-Mercaptobenzimidazole offers more than a mouthful to pronounce. The compound shows a bright, crystalline appearance with a mild, characteristic odor that some find slightly sulfurous. Research labs and industry put it to work in antioxidant systems in polymers, lubricants, and rubber. Its design—pairing a mercapto group’s reactivity with the benzimidazole’s versatile core—lends it resilience in processes that leave other molecules degraded or exhausted.
The compound’s molecular formula is C9H10N2OS. It weighs in at about 194 grams per mole, and typically melts between 172 and 175 degrees Celsius. Some batches I’ve handled formed colorless to slightly yellowish crystals; they dissolve in organic solvents like ethanol and acetone with little effort. Sparing water solubility curbs its reach in aqueous solutions, but that’s often an advantage for long-term stability. This molecule resists oxidation under standard storage conditions, thanks in part to the protective ethoxy group fending off unwanted side-reactions.
High-purity samples—over 98 percent, by HPLC or GC—prove critical for research and product applications. Labels detail batch number, purity, molecular structure, hazard warnings, and spec sheets. Usually, vials or drums bear hazard codes consistent with GHS and REACH standards, so anyone can quickly identify handling risks. The presence of the mercapto group calls for extra attention, as its reactivity could cause allergic skin responses in rare cases or interact with strong oxidizers. Temperature, humidity, and light sensitivity go on technical datasheets, so storage stays straightforward.
Synthesis starts with o-phenylenediamine and thioethers under controlled acidic or basic conditions. Ethylation of precursor compounds introduces the ethoxy group—commonly done with ethyl bromide or ethyl sulfate in a polar solvent. Purification leans on recrystallization, often from ethanol, to strip out trace impurities that could throw off results in downstream work. I have noticed that even tiny impurities can mask the true potency of the benzimidazole nucleus, especially in sensitive catalytic or pharmaceutical studies.
This compound reacts well with acylating agents, forming thioesters and new benzimidazole scaffolds. The sulfur atom supports further modification, sometimes acting as a nucleophile in ring closures or coupling reactions. In the lab, I’ve watched researchers tweak the ethoxy group or the benzimidazole rings to increase lipophilicity or biological activity. Its mercapto group can form disulfide bonds, unlocking more complex derivatives for industrial and biotech use. These modifications often mean better antioxidant power or entirely new therapeutic prospects.
Depending on the supplier or region, you might see it named as 5-Ethoxy-1H-benzimidazole-2-thiol, Ethoxybenzimidazolylthiol, or by company catalog product numbers like EBM-5 or BZIT-E5. CAS Registry Number 35561-28-5 remains the most consistent way to avoid mix-ups between similar molecules.
Personal experience and published research both agree: Direct skin or eye contact should be avoided. Gloves and goggles suit most handling needs. Labs install fume hoods when weighing or transferring grams to limit inhalation. If spillage happens, absorb with inert material and ventilate the workspace well. Waste disposal never cuts corners—residues enter hazardous chemical streams, and compliance with local, EPA, or REACH directives is non-negotiable. Safety sheets stress avoiding sources of ignition; this compound doesn’t ignite easily, but it can give off toxic fumes during decomposition. Training up younger chemists and technicians on these points keeps everyone and everything safer.
Polymer chemists use it to prolong the shelf life of plastics by halting oxidation. In lubricants, it keeps viscosity steady by stalling breakdown of hydrocarbon chains. Rubber manufacturers value its stabilizing effect—without it, many blends would fail under heat and wear. Analytical labs employ it as an internal standard, especially in NMR or HPLC studies. My favorite stories come from colleagues who tested this molecule as a corrosion inhibitor for metals, where even low concentrations kept rust at bay for months. It’s also gaining traction as an intermediate in high-value pharmaceutical syntheses, especially for drugs combating oxidative stress.
Current R&D trends stretch across multiple sectors. Material scientists see it as a foundation for designing new antioxidant systems that outperform traditional phenolic stabilizers. Specialists at the university level keep exploring therapeutic effects—chasing down hints of anti-inflammatory or neuroprotective properties. In medicinal chemistry groups, teams have toyed with both the ethoxy and mercapto groups, trying to reroute its activity and selectivity for even more specialized uses. Patents from the past decade point to a future where benzimidazole derivatives like this will appear in wound care, electronics, and herbicide screening. Following feeds from CAS and Google Scholar lets me spot new uses before they reach the market.
Toxicologists consider this compound of moderate concern if mishandled. Acute oral and dermal tests in rodents point to a high threshold for dangerous exposure—LD50 values run well above 500 mg per kilogram body weight. There’s little evidence of long-term carcinogenicity, mutagenicity, or reproductive impact in published animal studies, though every new analog gets re-tested. Human data is sparse, but routine use of personal protective equipment and ventilation remedies nearly all workplace risk. Waste streams avoid direct water or soil dumping, since bioaccumulation potential remains poorly understood. My work in compliance consulting always involves coaching labs to rely on up-to-date safety research; even a molecule with years of safe track record gets constant review.
The next decade looks promising for 5-Ethoxy-2-Mercaptobenzimidazole, as advanced polymers and medical compounds demand more specialized stabilizers. Its structure gives chemists room to bolt on new functions, from tailored ligands to imaging agents. The push for greener chemistry brings pressure to test it as a less toxic alternative to traditional stabilizers and corrosion inhibitors. I expect growing numbers of patents in electronics, coatings, and biopharma—especially as teams develop smarter tests to probe its subtle effects on living systems and the environment. With open sharing of research and ever-tighter regulation on chemical safety, this compound should play a bigger part in both everyday materials and next-generation medicines.
Run your hands along any reliable piece of industrial machinery and you’ll find a story few people realize. Metals working in engines, valves, or water pipes face a constant battle with corrosion. Here’s where 5-ethoxy-2-mercaptobenzimidazole steps in. Chemists recognized its knack for stopping corrosion on metal surfaces, especially for copper and its alloys, much like a protective barrier. Organic compounds like this often bring needed stability to parts used daily in power plants, electronics manufacturing, and even water treatment facilities.
Factories often deal with the challenge of copper corrosion, which leads to equipment failures or safety issues if left unchecked. Traditional corrosion inhibitors rely on older chemistries, some involving harsh environmental trade-offs. 5-ethoxy-2-mercaptobenzimidazole offers a balance: it forms a film on copper that blocks aggressive substances in water or industrial fluids from attacking the metal underneath.
I remember visiting a textile plant where bright green stains dotted the copper pipes. Turns out, those pipes ran hot water that accelerated their decay. The switch to a newer corrosion inhibitor containing 5-ethoxy-2-mercaptobenzimidazole brought those stains under control. Plant managers saw fewer breakdowns and saved both water and energy since equipment performed more efficiently.
Electronics make up one sector that leans on this compound. Printed circuit boards use copper tracks that need protection from oxidation during manufacturing and later, in devices people use every day. Compounds like 5-ethoxy-2-mercaptobenzimidazole help keep those copper traces clear, leading to longer-lasting and more reliable electronics.
In another part of industry, water treatment demands chemistries that do more than just break down sludge. Copper and other metals in heat exchanges, pipes, and valves need something that won’t just wash away with the water. This compound creates an adsorbed layer on metals. Without that, even clean water can eat away at metal over time.
With any specialty chemical, questions about health and environmental impact deserve attention. Scientific studies show that small concentrations of 5-ethoxy-2-mercaptobenzimidazole used in closed systems like power plant cooling circuits or as additives in lubricating oils don’t build up in harmful amounts. Responsible companies run risk assessments, limiting unnecessary emission or leaks. Regulatory frameworks demand proper labeling and handling, so operators aren’t left guessing about potential harm.
Protecting metals while reducing waste and chemical exposure often means tightening process controls. Automation and monitoring catch leaks or overuse fast. Research focused on green chemistry aims to deliver similar corrosion resistance with biodegradable compounds, but these solutions take time to develop and scale.
There’s a lesson here: the right inhibitor saves resources, keeps essential tools running, and protects workers and communities from unnecessary hazards. Better awareness and training ensure that modern corrosion management fits local regulations and global environmental goals.
Plenty of conversations about chemistry leave people lost in a sprawl of numbers and dashed lines. Among the avalanche of molecules, 5-Ethoxy-2-Mercaptobenzimidazole stands as more than a mouthful. This compound belongs to the benzimidazole family and has carved its niche thanks to specific modifications that tweak both its properties and uses. A molecule is not just a curious object for a laboratory shelf; ask anyone with a background in chemistry, and they know its structure spells out how it acts in real life—its reactivity, safety, and potential benefits or hazards.
5-Ethoxy-2-Mercaptobenzimidazole means the benzimidazole core carries a mercapto (thio, or –SH) group on position 2 and an ethoxy group (–OCH2CH3) on position 5. The skeleton starts as a bicyclic system—imagine double-ringed compounds like those found in caffeine or many drugs. Benzimidazole itself fuses a benzene ring to an imidazole ring. On that skeleton, an ethoxy group extends from the 5th carbon on the benzene ring, and a mercapto group attaches at the 2nd position of the imidazole ring.
Put differently, the molecule’s backbone lets it interact in ways simple rings never could. That thiol (–SH) group, in particular, interests folks in pharmaceutical development—for example, it can form bonds to metal centers, opening up a range of practical uses in corrosion inhibition, or biological functions if given the right conditions.
Years in academic labs taught me the difference between seeing a pretty ball-and-stick drawing in a textbook and actually synthesizing something. With chemicals like 5-Ethoxy-2-Mercaptobenzimidazole, you need to think through reactivity from that actual structure. The ethoxy group adds electron density, pushing electrons through the backbone and changing how the molecule reacts. That makes for stronger nucleophilicity at the sulfur compared to cousins without the ethoxy group. Not every chemical feature sits on a page—good safety goggles and a careful approach matter, since that mercapto group brings a particular odor and can react quickly with oxidizers.
Researchers have looked at similar benzimidazole derivatives to block certain enzyme activities or protect metals from rust. The modifications make these molecules versatile. My projects often relied on understanding these changes at the atom-by-atom level. Publications link benzimidazole derivatives to roles as antifungal agents, corrosion inhibitors, and even possible candidates in cancer therapies, because their structures wrap around biological targets with remarkable precision.
The important piece isn’t just reciting which element sits where—it’s seeing how a simple change in the molecule changes what you might do with it. Industry wants reliable corrosion inhibitors that do not leach harmful byproducts. Medicine searches for molecules that stop disease pathways. Every tweak to the skeleton—like placing an ethoxy group in just the right spot—can shift the possibilities. That’s where chemical intuition wins out, honed by time, mistakes, and lab notebook scribbles.
To make the most of discoveries around compounds like 5-Ethoxy-2-Mercaptobenzimidazole, experts need to keep explaining these chemical relationships plainly. Relying on solid research—peer-reviewed work, up-to-date analytical tools, and frank discussions about risks and ethical use—lays the groundwork for healthy progress. In the end, the right structure means the difference between another obscure lab sample and a material or medicine that actually changes lives.
5-Ethoxy-2-mercaptobenzimidazole doesn't often show up in everyday life, but anyone in chemical or pharmaceutical work might run across it. It’s part of the benzimidazole family, a group often used in manufacturing processes, lab research, and as intermediates in making more complex products like certain drugs or corrosion inhibitors. But just because it's not a household name doesn’t mean it can be ignored. Every chemical in a factory or a lab carries a story about risk, and it's worth taking a close look at this one.
With experience in chemical handling, I've learned that risk isn’t always about a gruesome warning label. It’s about the details. According to safety data and a deeper dive into the literature, this compound presents some notable hazards. Skin and eye irritation pop up as immediate risks if you get careless. Inhalation of dust during prep or handling can trigger respiratory problems. Benomyl, another compound from this chemical family, earned notoriety as a toxin. That raises a red flag, even though each related chemical has its own toxicity profile.
Long-term exposure brings up bigger questions. Some benzimidazole derivatives have links to liver damage and possible mutagenicity in animal studies. This doesn’t automatically throw 5-ethoxy-2-mercaptobenzimidazole into the “highly hazardous” pile, but it does steer us to caution. The kind of research I trust digs past the quick warnings. Even without clear classification as a carcinogen, the gap in human long-term studies means uncertainty holds real weight. Rushing to judgment in either direction would be irresponsible—especially if you’re handling kilos of the stuff day in, day out.
Unlike asbestos or cyanide, this chemical hasn’t grabbed headlines or been stamped with global bans. That lack of regulatory teeth sometimes tricks people into a false sense of security. Fact remains, many workplace incidents start with chemicals that seemed “low risk” until someone spent years breathing, touching, or getting them in their eyes. My practice in labs forced me to rely on more than just the absence of a major hazard symbol.
Current safety data sheets advise gloves, face shields, lab coats, and good ventilation when handling this chemical. Not overkill—basic respect, especially with a history of skin and mucous membrane reactions. Even trace contact can lead to allergic responses in people who are sensitive. There’s also the issue of environmental persistence; meaning, if it gets loose, it tends to hang around in soil or water longer than some compounds. This can spell trouble for wildlife, as aquatic organisms often prove more sensitive than people.
Hazard management isn’t glamorous. No one lines up to run drills on chemical spills or review waste protocols. Yet cutting corners because a chemical “hasn’t caused any problems yet” can backfire. Implementing chemical fume hoods, eye-wash stations, and strict spill management helps head off accidents. Keeping records of exposure, providing ample safety gear, and training workers on potential symptoms can save headaches—or worse—down the line.
Substitution sometimes offers relief. If a process can swap 5-ethoxy-2-mercaptobenzimidazole for a safer alternative, it’s worth running the numbers. Otherwise, attention to safe storage, routine monitoring, and careful disposal are the backbone of responsible use. For industries dealing in thousands of chemicals, risk never gets lower than the effort you put in.
At the end of the day, transparency and vigilance keep overlooked chemicals from becoming silent hazards. Whether regulations change or not, it’s always up to the people who use these substances to keep safety front and center.
I’ve spent years working with specialty chemicals in university labs and small manufacturing setups. Some folks glance over chemical storage protocols, thinking they only matter for the big-ticket stuff like acids or explosives. That kind of attitude leads to costly loss and health risks, especially with potent additives like 5-Ethoxy-2-Mercaptobenzimidazole.
This compound, used for its corrosion-inhibiting abilities and as a stabilizer in industrial processes, seems ordinary on paper. But any chemist will say that how a substance is cared for between uses marks the difference between reliable performance and a shelf of ruined product. No company or lab wants to explain why a batch failed, especially when it’s because someone cut corners on storage.
Let’s talk direct experience. Early in my career, a colleague ignored temperature guidelines for a similar heterocyclic compound, storing it near a heat vent. The next project kicked off with clumps and odd odors. The result: wasted hours and lost trust with the client who needed perfect samples.
5-Ethoxy-2-Mercaptobenzimidazole shows sensitivity to air and moisture. If you leave the container open or let humidity creep in, breakdown follows faster than you might expect. Even trace moisture causes clumping and leads to side reactions. The trick: use tightly sealed glass or plastic containers. Toss in a desiccant pack for good measure, and put the labeled jar in a cool, dry cabinet away from direct sunlight.
Direct sunlight or fluorescent lighting speeds up decomposition for plenty of organosulfur chemicals. I always treat light like a slow poison for anything with fragile rings or sulfur bridges. Opaque storage and shaded shelving don’t just save product—they keep your workspace safer.
Disposal deserves attention, too. After one project, a coworker washed leftover benzimidazole compounds down the drain, starting a headache for our facility manager. The right way? Collect waste in sealed, properly labeled bottles. Use your local hazardous waste program instead of household trash or sinks.
If the material heads offsite, it travels under strict labeling and packing. Secondary containment keeps accidental spills from leaving a mess all over the box truck or loading dock. It’s not just about fines or rules—nobody wants a call about harmful vapors escaping from a shipment long before it gets to its destination.
People overlook proper chemical management because it slows them down. My mentor taught me to set up storage as soon as a shipment arrives. Every time, the habit pays off through longer shelf life and more predictable results in experiments or applications. It even cuts down on panic when inspectors make surprise visits.
Product stewardship means looking out for everyone in the chain. By protecting materials from heat, light, and moisture, no surprises pop up during work, transport, or disposal. These extra steps help workers, end users, and the environment.
In my experience, it isn’t about memorizing more rules. It takes real responsibility and a little patience to safeguard chemicals as valuable as 5-Ethoxy-2-Mercaptobenzimidazole. The peace of mind beats the hassle every single time.
Ask any chemist about 5-Ethoxy-2-Mercaptobenzimidazole, and you might get a grin from someone who’s wrestled with stubborn reagents in the lab. This compound plays a supporting role in pharmaceuticals and corrosion inhibitors. Its presence may not jump out at most folks, but for anyone blending chemical solutions or developing new medications, knowing how it dissolves makes all the difference.
Water solubility tends to drive decision-making in research, industrial processes, and even environmental planning. Unfortunately, 5-Ethoxy-2-Mercaptobenzimidazole shrugs off water. Scientists classify it as practically insoluble in pure water—something I’ve seen frustrate even seasoned researchers. Handling stubborn powders like these drags down experiment pace, raises costs, and makes waste management tougher.
Organic solvents such as ethanol, acetone, and dimethyl sulfoxide step in here. This benzimidazole derivative dissolves more readily in these solutions. It forms clear mixtures in ethanol and DMSO at standard laboratory concentrations. I recall watching a colleague struggle for half an hour to disperse it in buffer—yet a splash of ethanol and the trouble vanished.
Higher temperatures help, but there’s a limit. Pushing things past 50°C sometimes brings diminishing returns and other side effects. Solubility, then, is not just a number—it’s a mechanical bottleneck in research and industry.
In pharmaceuticals, poor water solubility fences a molecule out from potential oral formulations. Bioavailability drops. Formulators working with 5-Ethoxy-2-Mercaptobenzimidazole must rethink delivery routes or use solvents or surfactants for injectable or topical forms. My time interning in a drug development lab taught me how rarely active molecules “just work” straight from the bottle. Teams ran solubility screens repeatedly, adjusting pH, temperature, and excipients until solutions settled.
The same issues surface in anti-corrosive coatings and specialty polymers, where the compound’s benefits hinge on even dispersion. Poor solubility creates weak spots and wasted materials, leading to costly recalls and safety risks. Environmental concerns also add weight, since undissolved particles can persist and bioaccumulate.
Instead of just working around the solubility struggle, industries now look at different strategies. Solubilizing agents and cyclodextrins make non-aqueous solutions more friendly to 5-Ethoxy-2-Mercaptobenzimidazole. Nanoformulation technology has turned into a real game-changer, breaking chemicals into tiny pieces that dissolve much more easily.
Open discussions about tough-to-dissolve chemicals like this also matter. Peer-reviewed journals and chemical suppliers have become more transparent about real-world results. Having navigated messy experiments more than once, I appreciate how honest data sharing knocks down research silos. By keeping a sharp focus on the actual numbers, not just optimistic catalog entries, more projects wind up on time and under budget.
There’s no single recipe for making 5-Ethoxy-2-Mercaptobenzimidazole play nice in every scenario, but a smart combination of good data, practical lab fixes, and solid collaboration makes the work a lot less frustrating. Lab notebooks across industries tell the real story—patience, careful tracking, and the right solvent at the right moment often steer projects straight.
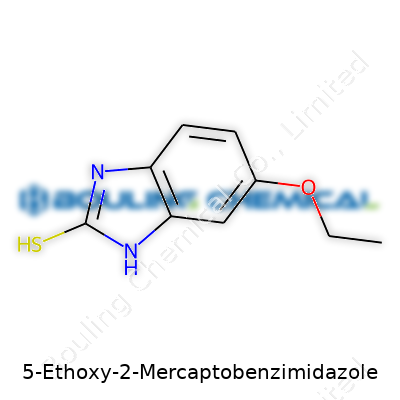
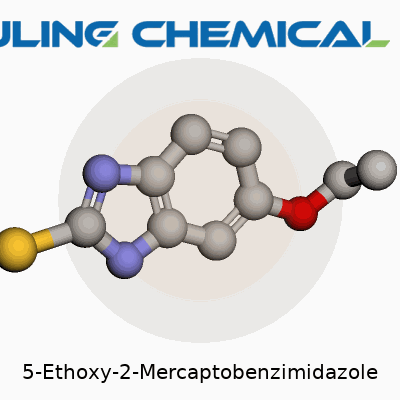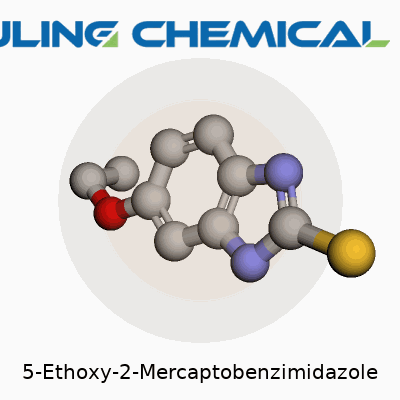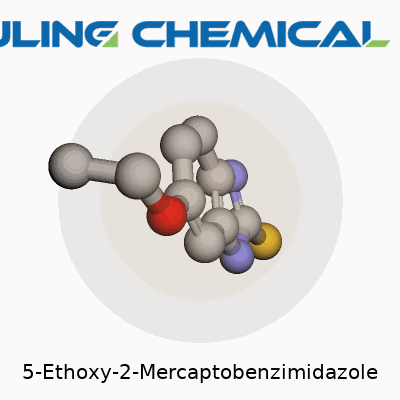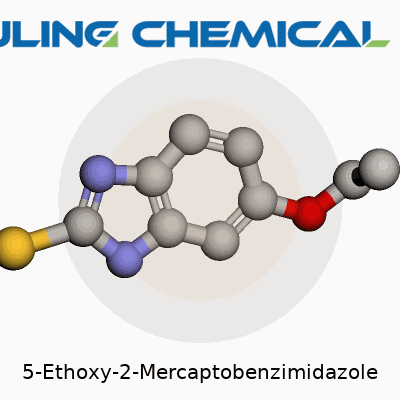
| Names | |
| Preferred IUPAC name | 5-ethoxy-3H-benzo[d]imidazole-2-thione |
| Other names |
2-Mercapto-5-ethoxybenzimidazole 5-Ethoxy-2-mercapto-1H-benzimidazole 5-Ethoxy-2-benzimidazolinethione |
| Pronunciation | /faɪ-ɪˈθɒk.si-tuː-məˈkæp.toʊˌbɛn.zɪˈmɪ.dəˌzoʊl/ |
| Identifiers | |
| CAS Number | [53807-57-7] |
| Beilstein Reference | 1643821 |
| ChEBI | CHEBI:138488 |
| ChEMBL | CHEMBL15079 |
| ChemSpider | 21162182 |
| DrugBank | DB08901 |
| ECHA InfoCard | 20a6fc9b-dc4c-4e85-8b3e-b92dfbf81afd |
| EC Number | 259-559-2 |
| Gmelin Reference | 108099 |
| KEGG | C14729 |
| MeSH | D000078641 |
| PubChem CID | 71872 |
| RTECS number | UU8225000 |
| UNII | 2C3S6T4X1K |
| UN number | Not classified |
| Properties | |
| Chemical formula | C9H10N2OS |
| Molar mass | 210.28 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.32 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.98 |
| Acidity (pKa) | 12.7 |
| Basicity (pKb) | 6.79 |
| Magnetic susceptibility (χ) | -62.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.736 |
| Dipole moment | 3.98 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 232.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -50.67 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -8375 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | A02BX02 |
| Hazards | |
| Main hazards | Causes skin and eye irritation. Harmful if swallowed. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 170°C |
| Autoignition temperature | 200 °C |
| Lethal dose or concentration | LD50 oral rat 2120 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 5-Ethoxy-2-Mercaptobenzimidazole: "LD50 (rat, oral) > 5000 mg/kg |
| NIOSH | PY8225000 |
| PEL (Permissible) | No PEL established |
| REL (Recommended) | 0.1 mg/m³ |
| IDLH (Immediate danger) | NIOSH has not established an IDLH for 5-Ethoxy-2-Mercaptobenzimidazole |
| Related compounds | |
| Related compounds |
5-Ethyl-2-mercaptobenzimidazole 2-Mercaptobenzimidazole 5-Methoxy-2-mercaptobenzimidazole 5-Chloro-2-mercaptobenzimidazole 5-Nitro-2-mercaptobenzimidazole 5-Ethoxybenzimidazole |