Curiosity has always fueled chemistry's greatest leaps, and the story of 5-ethenyl-2-pyrrolidinone grows from that drive. Years ago, chemists noticed the remarkable structure embedded in 2-pyrrolidinone and imagined adding a reactive ethenyl group would make for a molecule with powerful potential. The original syntheses came during a wave of research into pyrrolidinones in the early second half of the 20th century, as researchers searched for versatile intermediates that could bridge gaps between pharmaceuticals, polymers, and specialty chemicals. Patent filings from this era show that 5-ethenyl substitution captured attention for its capacity to influence reactivity and open doors in both academic and industrial labs.
5-Ethenyl-2-pyrrolidinone brings a reactive vinyl group to the classic five-membered lactam ring. This combination has made it more than just a niche chemical; it has become a foundational building block in several synthetic pathways. Chemists treasure this compound for its dual nature – the stability of a cyclic structure and the targeted reactivity of a vinyl group. Whether for advancing drug-like molecules or engineering new polymers, 5-ethenyl-2-pyrrolidinone shows up in patents, articles, and laboratory supply catalogs. As awareness of its properties grew, so did its uptake in various industries, prompting both custom synthesis and standard production lines.
Most chemists recognize 5-ethenyl-2-pyrrolidinone as a pale, often crystalline solid at room temperature, melting at moderate heat. It offers solubility in standard organic solvents – things like methanol, ethanol, chloroform, and DMSO all make reliable media for dissolving it. That vinyl group doesn’t just beg for attention; it makes the molecule ready for addition reactions, especially polymerizations and Michael-type reactions. Its ring stays stable under mild conditions, thanks to delocalization and resonance, so it doesn't degrade easily in storage, which is a relief for research and manufacturing alike.
Suppliers label 5-ethenyl-2-pyrrolidinone with its CAS number, 822-35-5, and provide data like purity, melting point, and key IR/NMR peaks. Most commercial lots top 98% purity. Labels give storage guidelines, usually cool and dry, away from light and oxidizers. Product sheets reference safety phrases from GHS labeling. Chemists familiar with the material keep Material Safety Data Sheets on hand. Documentation backs up traceability for use in regulated fields such as pharmaceuticals, where batch history matters as much as the end-use.
Lab-scale production of 5-ethenyl-2-pyrrolidinone often begins with 2-pyrrolidinone, using controlled halogenation at the 5-position, then follows with elimination to introduce the ethenyl moiety. Some processes take a more direct route from butyrolactam precursors, leveraging strong bases and controlled dehydrohalogenation. Academic journals describe improved variants that fine-tune yield through temperature, solvent, and order of reagent addition. In industry, continuous-flow reactors make the process more efficient, scaling from grams to kilos. The preparation method gets refined with every new patent, reflecting an ongoing quest for higher yield and convenience.
With the ethenyl handle, 5-ethenyl-2-pyrrolidinone invites modifications. Direct polymerization shapes it into specialty copolymers with applications in high-strength, water-soluble films. The vinyl motif enables cross-linking with acrylics, allyls, or styrenics, expanding utility for coatings and medical adhesives. Hydrogenation smooths out the ethenyl, producing more inert derivatives favored in certain medicinal syntheses. That lactam ring hosts redundant reactivity as well, with alkylation or acylation turning it into protected intermediates for richer chemistries. The versatility here hasn’t just helped research; it’s put 5-ethenyl-2-pyrrolidinone at the heart of several emerging patents for advanced materials.
Any chemical with commercial demand collects a spectrum of aliases. You’ll see 5-ethenyl-2-pyrrolidinone listed as N-vinyl-γ-butyrolactam, N-vinyl-2-pyrrolidone, or NVP in laboratory catalogs. Some vendors push branded versions, advertising “high-purity NVP,” “pharmaceutical-grade vinyl pyrrolidinone,” or “polymerization-grade NVP monomer,” reflecting the differing end-use needs from pharma to plastics. CAS numbers and standardized IUPAC names rescue buyers from confusion, as synonyms can lead to shipment mistakes in international supply chains.
Anyone handling 5-ethenyl-2-pyrrolidinone knows not to cut corners. Personal protective equipment is non-negotiable – gloves, goggles, and lab coats form the front line. Inhalation exposure brings irritation to respiratory pathways, and splashes risk eye and skin trouble. OSHA and REACH guidelines mandate proper labeling and disposal. Standard lab fume hoods provide ventilation, as the vinyl group means the compound can form reactive intermediates under the wrong conditions. Training makes a difference, and rigorous documentation supports audits in regulated environments.
Industries appreciate versatility. Pharmaceuticals exploit the lactam motif during the design of antimicrobial or CNS-active agents. Polymers use 5-ethenyl-2-pyrrolidinone as a monomer for water-soluble plastics with high binding capabilities—think tablet coatings, hydrogels for wound dressings, or adhesives. Specialty coatings and inks benefit from robust cross-linking, turning this compound into a foundation for products facing mechanical or chemical stress. More recently, researchers incorporated NVP into responsive materials used in sensors and biomedical diagnostics. The breadth of applications keeps research dollars flowing, as few molecules offer such flexibility at relatively low cost.
Academic teams and industrial scientists push the boundaries with every new project. Ongoing work explores green synthesis routes, such as solvent-free conditions or using renewable feedstocks. Catalytic pathways get investigated for energy efficiency and yield improvement, aiming for sustainability targets that regulators and consumers now demand. Analytical R&D delves into impurity profiles, seeking to back up claims of ultra-high purity for pharmaceutical lines. Teams design new copolymers leveraging the ethenyl motif, hoping to deliver materials that resist fouling, withstand sterilization, or mimic biological tissue. Patent filings reveal medical and cosmetic innovation, where formulations with 5-ethenyl-2-pyrrolidinone boast better bioavailability or controlled release. Every new experiment builds on decades of cumulative knowledge, setting higher standards for both material performance and environmental responsibility.
Work on the toxicology of 5-ethenyl-2-pyrrolidinone shows mixed results. In small-scale assays, acute toxicity remains low. Reports highlight mild skin or mucosal irritation at higher exposures, making safe handling protocols essential. Chronic or high-dose exposures haven’t triggered strong carcinogenic or mutagenic responses in standard animal studies, but documentation is careful not to declare the material hazard-free, especially in unventilated settings. Industrial hygiene watches for polymerization byproducts. Biodegradability studies show partial breakdown by soil bacteria, but aquatic environments degrade the molecule more slowly, so waste controls help protect drinking water and ecosystems. Published research keeps updating occupational exposure limits as new data surfaces, and companies invested in worker safety keep up with regulatory changes.
Every year brings new ways of thinking about multi-functional chemicals. As sustainability pushes gain traction, the future of 5-ethenyl-2-pyrrolidinone hinges on efficient, low-impact synthesis and expanded biodegradable applications. High-value pharmaceutical intermediates will drive continued innovation in stereoselective synthesis. Polymers built on this backbone target medical, electronic, and environmentally sensitive uses, where lightweight, robust, biodegradable materials compete for dominance. Many researchers anticipate the next decade will see machine learning and AI-driven design speed up the hunt for new NVP-based pharmaceuticals and functional materials. As regulations grow strict, producers adapt with greener chemistry and closed-loop recycling. The compound’s unique balance of chemical reactivity and manageable safety profile sets the stage for far-reaching discoveries, as engineers, chemists, and entrepreneurs bring ancient curiosity to today’s biggest scientific problems.
Sometimes, a molecule slips under the radar even though it does a lot of heavy lifting across several industries. 5-Ethenyl-2-Pyrrolidinone comes from the same family as some well-known chemical building blocks. It takes on a vinyl group, which means chemists can slot it into larger molecules or chain it together in polymers. This kind of structure gives it a certain flexibility and a talent for bonding that’s rare and valuable in the lab and on manufacturing lines.
Anyone who’s wrapped Christmas presents or opened a food package has handled plastics born from vinyl chemistry. 5-Ethenyl-2-Pyrrolidinone catches attention because scientists can use it to make specialty polymers. These aren't your everyday plastics; they get used for membranes, drug delivery tools, and materials that demand precise chemical properties. The substance helps control how flexible or water-loving a material becomes, all by locking in its chemical quirks during polymerization. Picture a contact lens or medical bandage designed to gently interact with the body—this molecule can be part of what makes that possible.
Big discoveries in medicine don’t just depend on the active ingredients. The way drugs dissolve, travel, or store on a shelf might owe a debt to hidden chemical players. Chemists use 5-Ethenyl-2-Pyrrolidinone to craft materials that trap drug molecules or change how fast medicine enters a person’s system. Its ability to add water-attracting traits to polymers lets formulators fine-tune drug delivery for both tablets and liquid medications. With patient comfort and safety always a top concern, chemists keep looking for ways to make medications safer and more effective. The right chemical helper can make all the difference.
With climate change talks heating up, finding new ways to clean air and water climbs the list of priorities. Water filtration relies on materials that soak up or repel certain contaminants. Some companies use compounds and polymers based on 5-Ethenyl-2-Pyrrolidinone for high-tech filtration membranes. These membranes sift out pollutants, separate out salts, or pull precious metals from industrial streams. Even energy storage tech, like batteries or supercapacitors, sometimes taps specialized polymers to control electrical flow and chemical stability. Every little improvement counts when scaling up clean energy or water purification worldwide.
Industry players have a responsibility to understand the risks and rewards of every ingredient they use. 5-Ethenyl-2-Pyrrolidinone, like many specialty chemicals, comes with its own safety story. Anyone handling or producing it needs solid training and up-to-date information on best practices. Research into alternatives or greener production routes ramps up as markets and regulators demand better. Companies investing in thorough safety reviews and open data sharing stand to build trust not just with regulators, but also with the communities they serve.
The future of advanced medicine, clean water, and better consumer products often starts with problem solvers in the lab. 5-Ethenyl-2-Pyrrolidinone stands as a testament to how much impact a single molecule can offer. By keeping applied research firmly rooted in safety and transparency, science can make sure benefits from these innovations reach people who need them most. The most promising solutions usually come at the intersection of curiosity, responsibility, and persistence.
Anyone who’s handled chemicals in a lab knows that storage habits shape both safety and the shelf-life of the product. 5-Ethenyl-2-Pyrrolidinone isn’t on every bench, but it crops up in research settings and industries working with specialty polymers or organic synthesis. It brings value to formulations, but it can stir up trouble if care drops.
In my time helping with lab audits and training new researchers, I’ve come to respect chemicals that seem ordinary at first glance. This compound reacts with air, light, or moisture—sometimes in subtle ways at first, but problems add up over weeks and months. A leaky cap or a warm shelf might go unnoticed, but spoilage or hazardous reactions aren’t so forgiving.
Storing 5-Ethenyl-2-Pyrrolidinone safely means getting a few basics right:
Labs that store everything on the same shelf run risks—mixing acids, bases, and organics as if labels were mere decoration. Cross-contamination invites fires, leaks, or ruined work. For this compound, I store it far away from strong acids, peroxides, and any oxidizers. Years ago, I opened a box of mixed bottles and found polymerized gunk where a careless hand had set organics beside bleach.
Everyone in the lab plays a role in chemical stewardship. Most problems vanish with a double-check on the container, attention to expiry dates, and swift cleanup of spills. At the organizational level, leadership can train staff routinely and invest in chemical tracking systems. Since industries using this compound often operate under regulatory eyes, following GHS guidelines and local chemical laws reduces both day-to-day hazards and long-term liability.
Working with specialty chemicals carries weight. Lax storage may cost a business lost product, liability, or health issues. Christening a fridge 'chemicals only' and setting rules for sealing and labeling avoided more messes than any emergency drill ever could. Safe handling preserves both materials and the people working with them, which sits at the center of good laboratory culture.
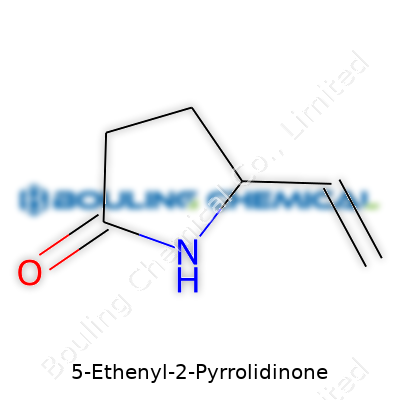
Thinking back to my days in the lab, understanding any molecule starts with its structure and formula. For 5-Ethenyl-2-pyrrolidinone, the chemical structure centers around a five-membered lactam ring, much like its sibling, N-vinyl-2-pyrrolidone, but with an ethenyl (vinyl) group on position 5. That simple swap of hydrogen for a vinyl group changes a lot. The molecular formula—C6H9NO—tells you it’s mostly carbon and hydrogen, with a nitrogen and one oxygen tagging along. You can picture a pyrrolidone ring, a familiar sight in polymers and pharmaceutical labs, now sporting a sharp, reactive vinyl tail.
This vinyl group isn’t decoration. It makes the molecule itch to attach itself to others. In practice, the presence of that ethenyl side chain transforms a pretty stable ring into something far more eager for chemical action. During my years working with polymer chemists, I saw how tiny tweaks like this opened up leagues of new uses. For example, the same chemical backbone with a different side chain gets snapped up for cosmetics, adhesives, or even drug delivery systems.
In 5-ethenyl-2-pyrrolidinone, the lactam ring (think N in the ring plus a C=O) supports the molecule’s stability, but that extra ethenyl group offers a reactive handle. Industry folks chase these handles: they become sites for polymerizing, for cross-linking, or grafting to surfaces. Material scientists look at these patterns and instantly start sketching possibilities.
Let’s stick to the evidence: Functional group chemistry makes or breaks usefulness. Literature confirms that adding a vinyl group to a lactam isn’t a shot in the dark—it ramps up the molecule’s reactivity, especially for forming polymers. These polymers can show up in water treatment, in medical coatings, or in specialized hydrogels. When you check the safety databases, you’ll see comparable backbone structures have already shown low toxicity and high solubility, both handy traits for things touching human skin or going inside the body.
Still, this kind of power doesn’t come without some baggage. Vinyl groups make the compound eager to react, which means shelf-life worries and the need for stabilizers. From experience, compounds like this can thicken or even gel unexpectedly—nobody likes finding their reactant turned into a solid plug overnight. A big issue comes with scale; labs can keep controls tight, but manufacturers face atmospheric oxygen and light, both of which can mess with vinyl chemistry.
Responsible handling means airtight storage, temperature monitoring, and a watchful eye on production lines. Researchers keep looking for stabilizers that do the job without getting in the way during processing.
Chemistry never stands still. Companies and labs keep tweaking side groups to pull the best out of these molecules. For 5-ethenyl-2-pyrrolidinone, improvements could come through safer, more stable formulations. Investment in green synthesis methods could reduce environmental impact, making production cleaner and safer. Open data sharing between academia and industry lets everyone move past the guesswork and take real steps toward safer, more reliable applications.
Innovation around smart storage, robust polymerization techniques, and biocompatibility will shape how far 5-ethenyl-2-pyrrolidinone goes. The structure draws chemists for a reason—it balances stability and reactivity in a way that keeps doors open for the next generation of materials and therapies.
5-Ethenyl-2-Pyrrolidinone might not sound familiar to most people, but its presence in labs and specialty manufacturing draws attention among those working with chemicals. This substance has a reactive vinyl group paired with a pyrrolidinone ring, which gives it certain useful properties—especially for creating polymers or specialty coatings. This also introduces concerns, especially regarding health risks and proper handling.
The question many have is whether this chemical poses hazards or toxicity threats. Most importantly, several studies flag irritation as a key concern. If someone accidentally gets it on the skin or in the eyes, redness or burning might appear quickly. Inhaling its vapors over extended periods raises the risk for throat irritation and headaches. These reactions are common for chemicals with similar reactive structures; the vinyl group often indicates some level of risk if safety measures are relaxed.
Workers in chemical processing tell similar stories: Without gloves or proper ventilation, minor mistakes can lead to stinging eyes or skin rashes. I’ve seen colleagues dismiss slight spills, only to need first aid minutes later, not realizing how quickly it can soak through thin materials or gloves.
Not many public long-term human studies focus on 5-Ethenyl-2-Pyrrolidinone. Looking at its chemical cousins, short-term effects usually top the list—skin, eye, and upper respiratory irritation. Animal testing shows certain doses could harm cell growth or cause mild toxicity signs, but nothing points to high-level, irreversible poisoning in limited short-term exposure.
The real risk climbs with frequency and amount. Chronic exposure, especially in poorly managed spaces, could affect lung function or skin health. Mistakes in labeling, accidental mixing, or ignoring storage instructions have caused chemical reactions, releasing irritating fumes. That points to a need for careful documentation and control, not just casual use or storage.
Regulators haven’t set sweeping bans or limits for 5-Ethenyl-2-Pyrrolidinone. Still, its listing in chemical safety databases means extra care in use and disposal. The European Chemicals Agency tracks it, urging users to employ personal protective equipment and avoid exposure. The US Occupational Safety and Health Administration references materials with similar hazards, and many chemical suppliers warn buyers about proper handling.
Chemical safety boils down to clear steps, not luck. Grab thicker gloves, goggles, and quality ventilation before even uncapping a bottle. Spill kits, washing stations, and clear labels need to live close to storage areas. Teams in manufacturing tend to run monthly drills—simple, routine checks that save money and health over time. Safety data sheets on file and open communication remove guesswork from the process.
Disposal often goes overlooked. Dumping leftovers down the drain or in regular trash risks environmental contamination and workplace fines. Specialized waste services make sure residues don’t leak into groundwater or municipal water supplies—a lesson learned after local reports found trace chemicals downstream from factory outlets.
Having worked with chemicals across labs and in industrial settings, the gap between safe use and danger often lies in human error. Respecting a chemical’s hazards isn’t just rule-following; it’s valuing the safety of everyone on site. By paying attention to early warning signs and using proper gear, risk drops enough that research or manufacturing can continue without avoidable emergencies.
Anyone who has spent time in a chemical lab or even a confined manufacturing floor knows the feeling that sinks in after hearing something shatter or tip over. One moment you’re focused on the task; the next, there’s a spill on the floor, and suddenly the air gets tense. With 5-Ethenyl-2-Pyrrolidinone, trusted knowledge and a bit of practice make all the difference. This compound, prized for certain polymer or specialty syntheses, comes with irritation or toxicity risks for skin, eyes, and lungs.
I find it pays to keep personal protective equipment close by, not hidden behind some clutter. The moment a spill happens, grabbing nitrile gloves, splash goggles, and a decent lab coat offers at least a fighting chance against burns or rashes. Sometimes people think a quick towel will do—until a patch of red, itching skin reminds them otherwise. Even after inhaling fumes, life shifts quickly from inconvenience to possible damage, especially for folks with allergies or asthma. A proper respirator or moving to fresh air can give lungs the break they deserve. The choice to take these basic precautions keeps panic and lasting harm at bay.
I remember a time watching someone racing for absorbent pads after a similar compound dripped from a tilted flask. Fast responses limit how far the liquid spreads. Pouring sand, vermiculite, or a lab-grade absorbent builds a barrier, making sure nothing snakes into a floor drain or seeps under equipment. Scooping or shoveling the mixture into a dedicated chemical-waste drum, then sealing it tight, means nobody else has to deal with residue or repeat exposure. If the room doesn’t air out fast enough, mechanical ventilation saves the rest of the crew from breathing irritation or unpredictable fumes.
Some people forget that wiping and scrubbing work better with the right cleaning agent. Using plain water risks a reaction. Mild soap solutions with plenty of rinsing get rid of invisible traces on skin and glass. Washing for at least fifteen minutes feels maddeningly slow during emergencies, but it truly prevents lingering irritation. If eyes catch even a splash, holding them open under a stream helps, just like running shoes under the tap after a muddy run. After every cleanup, reporting the incident logs a record for safety audits and helps the next person learn from the mistake, not repeat it.
Fresh faces in the lab often hesitate during their first emergencies. Good training, real drills, and dry runs help turn instinct into discipline. Reading safety data sheets should become a habit, not just an onboarding step. Keeping spill kits supplied and accessible, not tucked behind locked doors, empowers everyone working around these chemicals to act quickly. Encouraging a culture where people watch out for each other, speak up about missing gear, and raise concerns about risks prevents accidents from becoming disasters.
After handling chemicals for years, it becomes clear that the people using them shape the safety culture, not just the rules taped to the walls. Active communication, clear labeling, and a willingness to refresh skills keep everyone alert and prepared. By treating every spill or minor exposure as a chance to review what worked and what needs improvement, labs and workplaces build a stronger foundation—one ready for whatever tomorrow throws their way.
| Names | |
| Preferred IUPAC name | 1-Ethenylpyrrolidin-2-one |
| Pronunciation | /ˈfaɪ ɪˈθɛnɪl tuː paɪˌrɒlɪˈdiːnəʊn/ |
| Identifiers | |
| CAS Number | 22317-13-7 |
| Beilstein Reference | 187873 |
| ChEBI | CHEBI:155674 |
| ChEMBL | CHEMBL570123 |
| ChemSpider | 71343 |
| DrugBank | DB08797 |
| ECHA InfoCard | 03b8360e-6629-4289-88a3-4a6c3e6a1cc1 |
| EC Number | 220-392-5 |
| Gmelin Reference | 104328 |
| KEGG | C19673 |
| MeSH | D013471 |
| PubChem CID | 10401 |
| RTECS number | UY4375000 |
| UNII | 8N77O0X7V9 |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C6H9NO |
| Molar mass | 97.12 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | Odorless |
| Density | 1.088 g/cm³ |
| Solubility in water | Soluble |
| log P | -0.8 |
| Vapor pressure | 0.074 mmHg (25°C) |
| Acidity (pKa) | 12.4 |
| Basicity (pKb) | -1.02 |
| Magnetic susceptibility (χ) | -60.6·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.520 |
| Viscosity | 1.27 mPa·s (25 °C) |
| Dipole moment | 3.90 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 294.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –35.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -340.9 kJ·mol⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07, GHS05 |
| Signal word | Danger |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P273, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-2-0 |
| Flash point | 96 °C |
| Lethal dose or concentration | LD50 oral rat 430 mg/kg |
| LD50 (median dose) | LD50 (median dose): 373 mg/kg (Rat, oral) |
| NIOSH | MV3850000 |
| PEL (Permissible) | No PEL established. |
| REL (Recommended) | REL (Recommended): 0.05 ppm (0.15 mg/m3) |
| IDLH (Immediate danger) | No established IDLH value. |