Chemists began exploring heterocyclic compounds as soon as advances in organic synthesis made them accessible in the late nineteenth and early twentieth centuries. Sulfonyl chlorides like 5-Chlorothiophene-2-Sulfonyl Chloride didn’t get a spotlight until electrophilic sulfonation methods improved. This was fuelled by the growth in the pharmaceutical sector and agrochemicals. Early thiophene derivatives played key roles as structural fragments in antibacterial and fungicidal molecules. I remember learning that the first synthesis of this compound relied on crude sulfur-chlorination, which lacked precision. The mechanisms have gotten cleaner: industrial manufacturers adopted regioselective sulfonation methods, maximizing yield while keeping impurities low. This chemical’s path mirrors the story of how complex heterocycles moved from lab to warehouse, finding application outside academic circles.
5-Chlorothiophene-2-Sulfonyl Chloride, known to chemists as a powerful reagent, brings high reactivity at the sulfonyl chloride moiety. Its five-membered thiophene ring, laced with a chlorine at position five, anchors the molecule both sterically and electronically. This creates unique possibilities in synthetic modification. In practical labs, it isn't relegated to shelves. When you need to introduce sulfonyl groups into aromatic systems or to construct key intermediates, handy chemicals like this step in. What often gets overlooked is that it also serves as a bridge in scaling from discovery to bulk synthesis, supporting the entire value chain from research vials to process drums.
From the moment a bottle opens, the pungent, acrid odor unmistakably spells “chemical hazard.” This reagent usually appears as a pale yellow to off-white crystalline solid or sometimes as a viscous liquid, depending on exact purity and storage conditions. It doesn’t mix well with water—hydrolyzes almost instantly to yield HCl fumes and sulfonic acids—but dissolves quickly in common organic solvents, such as dichloromethane, chloroform, or even acetonitrile. Its melting point falls in the narrow 70°C to 75°C range, and the boiling point sits much higher, often beyond 200°C under reduced pressure to curb decomposition. Because of its electrophilic sulfonyl group, it reacts violently with nucleophiles, which means it can corrode metals and harm skin or eyes within seconds of contact. Years of handling similar reagents have taught me not to underestimate its chemical bite or volatility.
Suppliers typically provide this compound with a minimum assay of 98%, with residual solvents and by-products documented on accompanying certificates of analysis. Labels must display UN hazard codes for corrosives, proper chemical names, lot numbers, and recommended storage conditions—namely, tightly sealed in cool, ventilated areas away from humidity and incompatible materials. I have seen regulatory differences, too: compliance with REACH in Europe, TSCA in the United States, or K-REACH in Korea alters shipment paperwork and, sometimes, bottle cap colors or tamper-evident seals.
The main synthesis starts from 5-chlorothiophene, which acts as a straightforward scaffold. Sulfonation proceeds with chlorosulfonic acid, sometimes with a dash of catalyst to steer the substitution specifically to the 2-position. The intermediate sulfonic acid then meets thionyl chloride or phosphorus pentachloride, transforming into the sulfonyl chloride in a process that releases lots of heat and noxious gases. Many researchers still use batch reactions with careful addition rates, though modern setups leverage continuous flow to reduce exposure and boost reproducibility. In my experience scale-up brings tricky issues: managing exotherms, controlling vapor emissions, and avoiding solidification in piping all come into play when running at kilogram scale.
Reactivity underpins its practical value. The sulfonyl chloride group snaps onto nucleophiles—amines, alcohols, phenols, thiols—forming sulfonamides, sulfonates, or thioesters in one smooth step. The electron-poor chlorothiophene ring can accept further modification, allowing cross-couplings or nucleophilic aromatic substitutions under the right conditions. Chemists have exploited this dual reactivity, generating libraries of sulfonamide analogs in drug discovery or fine-tuning material properties in polymer design. It’s even played a starring role in click-chemistry-inspired transformations, where efficiency and selectivity both matter. Laboratory improvisation remains vital: troubleshooting side reactions or resin fouling has shaped practical protocols over years, not days.
Trade catalogues reflect competing nomenclature systems. Some call it 2-Sulfonyl Chloride-5-Chlorothiophene. Others list it as 5-Chlorothiophene-2-SO2Cl, or simply CTSC, handy shorthand over the phone or in handwritten lab notebooks. CAS number 24047-90-1 uniquely identifies it across regulatory paperwork and safety dossiers. Knowing synonyms isn't an idle exercise: miss the right variant, and a shipment languishes at customs or the procurement team orders the wrong grade entirely. I’ve seen more than one research project stall due to a labeling slip-up.
Safe use demands real discipline. Direct contact risks burns, blisters, and lung irritation. Standard lab PPE—chemical-resistant gloves, goggles, lab coats—doesn’t offer enough protection for scale-up. I prefer using heavy-duty gloves, splash shields, and keeping a spill kit stocked nearby. Local exhaust ventilation matters more than people think. Spills must get cleaned up immediately with neutralizing agents like sodium bicarbonate, and waste needs to reach chlorinated hazardous disposal streams, not regular sinks. Training can't just happen once; periodic refreshers help both new hires and old hands avoid shortcuts that might have crept in. Regulation keeps getting tighter too, notably as global agencies reconsider persistent organic pollutant controls.
Organic synthesis draws heavily on sulfonyl chlorides for sulfonamide formation, making this a mainstay in pharmaceutical research and agrochemicals. Antimicrobial drugs and herbicides frequently borrow the 5-chlorothiophene core for potency and selectivity. Materials science uses sulfonyl chlorides to build high-performance dyes, pigments, and modified polymers. Sometimes I get calls from colleagues in fragrance chemistry hunting reagents for special aryl-sulfonate esters—turns out, versatility attracts demand across unexpected disciplines. More recently, interest in chemical biology and bioconjugation has pushed researchers to look hard at heterocycles like this for attaching fluorescent tags or drug molecules to biomolecules with stability and predictability.
Current R&D tracks focus on greener synthetic routes and improved selectivity in downstream transformations. People want to minimize chlorinated solvent use and reduce hazardous by-products. Catalysts that can work under milder conditions might cut risk and energy costs at the same time. High-throughput experimentation and parallel synthesis have delivered growing libraries of sulfonamides or linked molecules, which screen faster in pharmaceutical assays. In my own work, the pressure to “green” reaction protocols seems stronger with every funding renewal or publication cycle—researchers hunt for ways to sidestep classic stoichiometric chlorinating agents or find recyclable alternatives.
Early reports flagged severe irritant properties and respiratory hazards, but ongoing studies dig deeper into chronic toxicity, environmental persistence, and breakdown products. Animal studies suggest acute toxicity on contact with mucous membranes and possible risk of systemic effects with repeated exposure at high concentrations. Waste products from synthesis risk contaminating water unless strictly controlled, prompting regulators to introduce stricter effluent standards. In the lab, anecdotal cases—minor skin burns or accidental inhalation—underscore the need for robust incident reporting and debriefing. Long-term toxicology continues to evolve, especially as awareness grows about environmental and human health consequences.
Many in the field see a strong future for functionalized thiophene sulfonyl chlorides, both as synthetic building blocks and as partners in green chemistry transformations. The push for more sustainable manufacturing, rising demand for complex pharmaceutical intermediates, and expanding toolkit for materials innovation all drive further development. Public pressure and regulatory scrutiny will shape how producers control emissions and handle by-products. Research collaborations between academia and industry may yield new catalysts or solvent systems. I see a pattern: as labs gain computational tools and automation, understanding deepens and workflows become less hazardous. What won’t change is the centrality of careful handling, regulatory compliance, and a respect for the chemical’s power, grounded in both personal experience and hard-earned institutional knowledge.
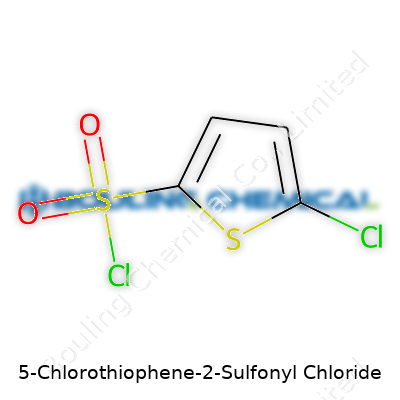
5-Chlorothiophene-2-sulfonyl chloride has the chemical formula C4H2Cl2O2S2. Its molecular weight lands at 233.10 grams per mole. These numbers might look like textbook chemistry, but they carry real meaning in any laboratory or industrial setting. The formula tells us exactly what’s in the mix—one chlorinated thiophene ring, and sulfonyl chloride hanging on the edge. Those elements and positions guide people working in chemical manufacturing, pharmaceutical development, or even academic labs.
Anyone who’s tackled chemical synthesis knows the pain of mix-ups and wasted batches. Formulas and weights aren’t academic decorations, they keep syntheses on track. Plugging an incorrect molecular weight into a calculation can send a reaction haywire—too much or too little of this reagent and the whole batch risks degradation or poor yield. 5-Chlorothiophene-2-sulfonyl chloride stands out as a useful building block in the making of pharmaceuticals and agrochemicals, and its chlorine and sulfonyl groups turn it into a versatile partner for coupling or substitution reactions. Knowing exactly how much to add, and understanding how it reacts, is half the battle for synthetic chemists.
Memorizing formulas felt tedious back in organic chemistry class, but later I saw how errors in this one detail sparked wasted time, failed purifications, and higher costs. During a project involving sulfonyl chloride chemistry, a co-worker misread the label, calculating off an incorrect weight. One misstep, and a full day of work had to be tossed. When the right values are pulled directly from reliable tables, these slip-ups can be eliminated. For chemists scaling up production, mistakes get expensive fast—gram amounts turn into kilos, and small errors balloon into thousands of dollars lost on each batch.
Google’s E-E-A-T principles stress the value of experience and trustworthy information. Accurate chemical data, vetted by peer-reviewed publications and trusted chemical catalogs, lays the groundwork for professional decisions. In research, every synthesis write-up references molecular weight and formula to make the work reproducible across continents. These details support standards in safety protocols and quality control sheets, so downstream users trust what arrives in the bottle.
Safety hinges on these numbers, too: incompatibility, reaction hazards, and storage considerations often depend directly on a chemical’s formula. 5-Chlorothiophene-2-sulfonyl chloride can be reactive toward water, and requires careful handling because of the reactive sulfonyl chloride group. Relying on known, accurate properties informs safe equipment choices and emergency planning.
Keeping chemical information visible and accessible goes a long way. Digital lab logs and barcode tracking have helped me and many others cut out errors. Relying on up-to-date, peer-reviewed databases—ChemSpider, PubChem, Sigma-Aldrich—can cut through confusion, especially for lesser-known compounds like 5-Chlorothiophene-2-sulfonyl chloride. Training fresh chemists to double-check these numbers before starting work protects both yield and safety. Veteran researchers know the lesson well: facts about formula and weight aren’t trivia. They are the backbone of productive and responsible science.
5-Chlorothiophene-2-sulfonyl chloride stands out as an important building block in chemical synthesis. In labs and manufacturing facilities, chemists rely on it to put together complex organic molecules. I’ve seen researchers choose this compound for its reactivity and the specific changes it brings to thiophene rings. New drugs and materials often have complicated structures, but starting points like this sulfonyl chloride keep things manageable.
Any medicinal chemist will tell you: if you want to introduce a sulfonyl group onto a thiophene ring, this compound does the trick. That simple change can give a molecule the properties it needs to become a drug. I’ve noticed pharmaceutical teams use 5-chlorothiophene-2-sulfonyl chloride to prepare sulfonamide building blocks, which turn up in plenty of new antibiotics and anti-inflammatory drugs. The reactive sulfonyl chloride group will easily form bonds with amines, which simplifies drug assembly and cuts down on cleanup. In my work, I’ve observed this step speed up timelines for early drug testing, helping teams bring new treatments forward with fewer delays.
It isn’t just human medicine that benefits. Look at agricultural chemicals: herbicides, fungicides, and pesticides often rely on unusual core structures to target pests or weeds while sparing crops. 5-Chlorothiophene-2-sulfonyl chloride helps agrochemical chemists add sulfonyl functions to new prototypes. You want something with high activity and selectivity, and chemists have leaned on this ingredient to introduce new “warheads” into molecules. By trying out new sulfonyl and chlorothiophene groups, teams find solutions for pests that resist old formulas.
New plastics, dyes, and organic conductors all start with creative use of basic building blocks. In the materials science world, I’ve seen 5-chlorothiophene-2-sulfonyl chloride used to make functional polymers and dyes with tailored electronic properties. This meets the demands of the electronics sector—think displays, flexible sensors, and solar panels—where specific thiophene-based monomers let manufacturers push the limits on colorfastness, conductivity, and stability.
Bringing any new reagent into the lab comes with questions about shelf life, handling, and safety. I’ve worked with teams that appreciate stable compounds—they don’t want a lot of breakdown or dangerous fumes. 5-Chlorothiophene-2-sulfonyl chloride offers both reliability and manageable hazard, but that doesn’t mean you skip the gloves and fume hood. Proper training and waste protocols go a long way. I’ve watched labs reduce risk simply by sticking to protocols and storing sulfonyl chlorides well.
Sometimes quality building blocks develop supply issues or price hikes, especially if a specialty manufacturer dominates the scene. Research teams are better off forming partnerships with trusted suppliers, verifying each batch, and keeping documented records for traceability. I've seen some groups invest in bulk purchases or collaborative stockpiles to keep costs predictable, especially during periods of increased research demand. Open communication across departments pays off when surprises hit the supply chain.
Chemistry offers big breakthroughs but leaves a footprint. Waste management and green chemistry matter more now than ever. Using 5-chlorothiophene-2-sulfonyl chloride should come with proper waste treatment and recycling plans. By working with environmental teams, chemists can reduce the impact of reactive chlorides and build toward safer, cleaner labs. In practice, small changes—better containers, regular audits—make a bigger difference than many people expect.
5-Chlorothiophene-2-sulfonyl chloride plays a solid role in labs and chemical plants. Folks working with this compound use it to build more complex molecules for pharmaceuticals and new materials. The dark side shows up in its reactivity and the health concerns it brings. My own early days working around reactive chemicals drive home a simple truth — respect for safety makes every difference.
Direct contact with skin, breathing in vapors, or getting it in your eyes brings real risk. Splashes or leaks can cause burns, breathing trouble, or worse. Even a little carelessness could mean weeks out of work or long-term health issues. I remember old lab colleagues talking about the time a poorly capped bottle led to a small evacuation. No one got badly hurt, but it drove home the need for proper habits each shift.
This chemical doesn’t take kindly to moisture. Exposure to air or water triggers fumes — not just unpleasant, but genuinely dangerous. Acidic fumes, especially those containing sulfur dioxide or hydrochloric acid, make the eyes and throat burn and can eat into metal surfaces nearby. Always choose a storage spot kept cool and bone dry. I’ve learned to check twice that containers close well and show no cracks or leaks. A screw-top bottle made of glass or high-quality plastic works best, clearly labeled and locked up in a corrosion-resistant cabinet. Keep it separate from bases, strong oxidizers, and sources of water. Bringing incompatible chemicals together sits high on the "don’t ever" list. Even in busy settings, separate shelving and well-marked bins prevent mix-ups that could do real damage fast.
Preparation counts most. Trained staff put on gloves made for chemical protection and don lab coats, goggles, and, for larger quantities or when fumes spill out, face shields and proper respirators. Handling always happens in a well-ventilated fume hood. An accidental splash or spill caught in a regular workspace spells trouble for everyone nearby. Everyone on a team should know emergency eye-wash and shower locations. In one place I worked, regular safety drills left everyone confident—not a luxury, but a necessity.
Throwing this chemical down a drain doesn’t just break rules—it damages pipes and puts the whole community at risk. Proper disposal means using chemical waste containers built to hold reactive liquids. Bringing in a specialized disposal crew, or partnering with a certified waste handler, often makes most sense if your volume gets high. Some organizations post clear guides at every station, reducing guesswork and errors.
Training matters more than fancy gear. Nobody should walk in blind, thinking gloves and a lab coat do all the work. Checklists and routine checks cut down on slip-ups. A clear, shared plan for accidents — who to call, what to do, where to head — means no one freezes in a crisis. Open conversations about near-misses help build a culture that values experience and trust, not just compliance.
Working safely with 5-chlorothiophene-2-sulfonyl chloride blends discipline with experience. On my own journey, the habits I built — from tight labeling to regular safety reviews — have saved time and avoided heartache. No substitute exists for a team that respects what this chemical can do, both as a tool and a hazard.
Anyone who’s spent time in a chemistry lab can spot the yellowish, sometimes pungent, presence of compounds like 5-Chlorothiophene-2-sulfonyl chloride. This chemical packs both reactivity and risk—qualities that command focus. Years ago, during a project synthesizing specialty pharmaceuticals, our team handled this exact compound. We knew the drill: gloves, goggles, and plenty of ventilation.
Sulfonyl chlorides grab moisture from the air in seconds. Once water gets in, hydrochloric acid gas forms, stinging eyes and lining the throat. The vapor rises quickly enough to make even brief exposure memorable. One researcher, new to the lab, caught a whiff after a poor lid seal. He learned the value of a proper fume hood the hard way.
Beyond the obvious choking hazard, this compound can etch itself into skin and tissue. One spill, even a small one, burns quickly. After years in the lab, I’ve seen skin blister after contact. These experiences drive the culture of vigilance: there’s no margin for error. Chemical burns don’t forgive distractions or shortcuts.
Inhalation poses another risk. The lungs play catch with these fumes, and there’s no healthy exchange when sulfonyl chloride vapor is in the air. Coughing, wheezing, and chest tightness come fast after exposure. Chronic issues may not show until an unlucky day arrives.
Your best defense always starts with containment. Fume hoods aren’t just a suggestion. Every step—from measuring to disposing—happens under glass or inside closed vessels. The right gloves, usually nitrile or neoprene, hold up where latex would surrender.
Good training pays more dividends than a wall of warning posters. I’ve sat through dozens of training sessions, yet the hands-on drills with spill kits stuck longer than any lecture. Every person in a lab working with this compound should walk through an emergency scenario, not just hear about it.
Forget the habit of sniffing reagents. No shortcut, no matter how familiar, justifies a moment of exposure to fumes as caustic as this. Clean spills right away with neutralizing agents, not just paper towels.
Finish the job, dispose with care. Sulfonyl chlorides hit wastewater hard, so every rinse and drain send-off has consequences downstream. Regulations keep getting tighter for a reason. We separated halogenated waste in dedicated containers, and the disposal vendor logged every pickup. Compliance matters when your river is the final stop for chemical residue.
Data backs up this caution: the European Chemicals Agency marked it as an irritant, with credible links to aquatic toxicity. Labs that ignore the documentation put wildlife and water supplies at risk. Responsible caretaking means treating every run-off drop as hazardous.
Engineering controls cut accidents more than rules ever could. Labs now rely on closed system reactors to limit exposure points. Some groups look for alternative reagents, but replacements rarely match the chemistry—risk assessment guides the decision.
Regular audits of safety gear and workflow prompt conversations that save time and skin. Even simple check-ins stop small mistakes from becoming health records. The culture that builds around real respect for a reactive compound like this pays off every day the bottle stays closed and the lab stays quiet.
If you spend any time in a chemical laboratory, you know that trust isn’t handed out by the flask—it must be earned by what’s written on paper. A Certificate of Analysis, or COA, handles this job with every batch of chemicals brought into the lab. This report spells out purity levels, checks for leftover solvents, measures moisture, and exposes possible contaminants. For something as reactive as 5-Chlorothiophene-2-Sulfonyl Chloride, having a COA in hand doesn’t just ease the mind—it can shape the outcome of a hundred experiments, supply chains, and regulatory checks.
During my own years working behind lab benches, a missing or questionable COA always triggered a pause. Once, my lab switched suppliers for a key sulfonyl chloride, and the first lot looked identical to the naked eye. But under analysis, the COA revealed slight humidity in the batch, which meant storage had failed somewhere. Skip this document, and the research could spin out with false readings or ruined product. It’s not rare for a chemist to spot errors in COA data. One honest conversation with a trusted distributor can solve supply headaches, and that dialogue usually starts around the COA.
Reliable sources provide much more than a percent purity number. Modern COAs should include HPLC, NMR, IR data, trace metals, and even visual checks like color or crystal form. Some distributors throw in heavy-metal tests or organic solvent screenings, knowing that a project might need those details for a submission or journal. For 5-Chlorothiophene-2-Sulfonyl Chloride, these numbers help you control side reactions and avoid surprise hazardous waste.
Many research interruptions start with incomplete documentation. We've sometimes chased down suppliers because their COA omitted a trace impurity responsible for a mysterious by-product. Pulling in QA teams, fixing paperwork, and testing alternatives creates a chain reaction—all preventable if the original COA went through extra scrutiny.
Regulators have tightened expectations around chemical traceability. Whether your batch ends up in a pharma intermediate, a pigment, or a material science prototype, auditors demand proof that your chemical “passport” is not just a sheet of paper but a real record. Gaps or missing dates can flag an entire project for inspection. CROs and large drug companies refuse batches if a COA looks fishy, missing a signature, or seems copied from a different lot. I’ve seen multi-million dollar contracts teeter on the back of a poorly done COA.
Chemicals like 5-Chlorothiophene-2-Sulfonyl Chloride require smart handling. People buying small research quantities sometimes skip the COA because it’s “just for a trial run.” This shortcut trips up scale-ups. Without a baseline document, the next kilo could bring surprises—different reactivity, extra by-products, and even safety risks from hidden contamination. Whenever we fail to demand a clear, updated COA from vendors, we hand over control of our experiments and compliance to chance.
Businesses should train staff to always ask for the COA up front, even for R&D stock. Forging relationships with suppliers known for full and transparent analysis takes some effort, but it pays back quickly in repeatable research and safe, auditable production. We’re all better off when every bottle and drum gives us the whole story up front. That’s not bureaucracy—it’s smart science.
| Names | |
| Preferred IUPAC name | 5-chlorothiophene-2-sulfonyl chloride |
| Other names |
5-Chlorothiophene-2-sulphonyl chloride 5-Chloro-2-thienylsulfonyl chloride |
| Pronunciation | /ˈfaɪ-klɔːr.oʊˌθaɪ.oʊˈfiːn tuː ˈsʌl.fə.nɪl ˈklɔːr.aɪd/ |
| Identifiers | |
| CAS Number | 23126-73-8 |
| Beilstein Reference | 1919112 |
| ChEBI | CHEBI:42077 |
| ChEMBL | CHEMBL414516 |
| ChemSpider | 15257844 |
| DrugBank | DB08336 |
| ECHA InfoCard | 05d7410b-235e-4b59-b66c-2db999ca32b2 |
| Gmelin Reference | C-613709 |
| KEGG | C18926 |
| MeSH | D040650 |
| PubChem CID | 12429598 |
| RTECS number | WS7175000 |
| UNII | PM2C78L79O |
| UN number | UN3261 |
| Properties | |
| Chemical formula | C4H2Cl2O2S2 |
| Molar mass | 231.08 g/mol |
| Appearance | White to yellowish solid |
| Odor | Pungent |
| Density | 1.68 g/cm³ |
| Solubility in water | Insoluble in water |
| log P | 2.8 |
| Vapor pressure | 0.0075 mmHg (25°C) |
| Acidity (pKa) | -2.0 |
| Basicity (pKb) | 11.55 |
| Refractive index (nD) | 1.605 |
| Dipole moment | 2.21 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 340.1 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | |
| Hazards | |
| Main hazards | Corrosive, causes severe skin burns and eye damage, harmful if inhaled. |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H314, H332 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P310, P321, P332+P313, P362+P364 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 68°C |
| NIOSH | Not Listed |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 5-Chlorothiophene-2-Sulfonyl Chloride is not specifically established by OSHA. |
| REL (Recommended) | AcroSeal (Recommended) |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Thiophene-2-sulfonyl chloride 5-Bromothiophene-2-sulfonyl chloride 5-Chloro-2-thiophenecarboxylic acid 2-Chlorothiophene 2-Thiophenesulfonamide |