A quick glance through chemisty journals from the last century reveals a constant hunt for new ways to modify and use heterocyclic compounds. The story of 5-Chlorothiophene-2-Sulfonamide feels like a thread running through that wider tapestry. Thiophene itself cropped up in the 19th century, nabbed from coal tar as curiosity, but synthetic tweaks started rolling right after folks realized its potential for medicine and materials. Researchers tested out substitutions on the thiophene ring throughout the 1900s. The 5-chloro flavor, plus that sulfonamide group, started drawing real notice in the 1970s as advances in analytical tools made these molecules easier to isolate and characterize. Chemists with an eye for pharmaceutical applications spent years teasing out structure-activity relationships. By the 1980s, several research streams pointed at 5-Chlorothiophene-2-Sulfonamide both as a standalone building block and a handy intermediate for taking molecules in promising new directions.
It’s easy to get distracted by dazzling new chemical names, but 5-Chlorothiophene-2-Sulfonamide represents a tidy piece of work: a thiophene ring, decorated at the 2-position with a sulfonamide group, at the 5-position with chlorine. It's not shouting for attention, but it stays flexible. Agents like this don’t always headline drug pipelines or industrial roadmaps, but they keep the wheels turning in labs and pilot plants by enabling rare and subtle reactivity.
Pull a sample of 5-Chlorothiophene-2-Sulfonamide from a well-stocked shelf, you'll likely get an off-white to pale beige powder. The melting point usually lands between 110 and 120°C. The presence of a sulfonamide makes it decently soluble in polar organic solvents; dimethylformamide or DMSO both handle it well. Water solubility sits lower but isn't out of reach, driven by that polar SO2NH2 side chain. Reactivity climbs out of the electron-withdrawing chlorine and sulfonamide groups, which nudge the reactivity of the thiophene ring, setting up selective transformation opportunities. That subtle balance draws in chemists.
Suppliers often tag the product with purity ratings at or above 98%, tracking common impurities by HPLC or GC-MS. Structural confirmation rides on NMR and IR signatures. Proper labeling follows all chemical regulatory norms, from GHS hazard pictograms to lot numbers and batch-specific analysis sheets. The molecular formula keeps to C4H4ClNO2S2, molecular weight usually clocks in at 213.68 g/mol. Labels flag IUPAC and CAS registry data for traceability; nothing gets left to chance on compliance paperwork. Package labeling stays clear to avoid mix-ups in high-throughput environments.
In the lab, a common route starts with 5-chlorothiophene, which undergoes sulfonation to yield the sulfonic acid intermediate. From here, conversion to the sulfonyl chloride opens the door for direct amidation, dropping in an ammonia source to complete the sulfonamide piece. The whole job requires patient temperature control, precise addition rates, and careful quenching, since both thiophene substitution and sulfonamide formation play by their own rules. Well-ventilated fume hoods stay critical, because intermediates can release volatile byproducts that wreak havoc indoors. Industrial processes run similar schemes but jack up scale and automation, often closing off the reaction streams to minimize operator risk and boost yields.
The 5-chloro and 2-sulfonamide gates on the thiophene ring set up a toolbox for selective reaction. Nucleophilic aromatic substitution works at the 5-chloro mark, especially with strong nucleophiles at a decent temperature bump, opening doors to a wild variety of derivatives. That SO2NH2 group adds another layer: chemists can tack on protecting groups, pop off the NH2 for other functionalities, or use it as a handle for bioconjugation. Oxidation and reduction events aim directly at the thiophene system, attuned to the substituent effects. The structure can serve as a stepping stone, rather than a final product, in multi-stage syntheses targeting everything from agrochemicals to diagnostic agents.
Buyers and researchers know this molecule by a crowd of labels: 5-Chloro-2-thiophenesulfonamide, or just 5-Chloro-2-Sulfonamidothiophene, or sometimes Thiophene-2-Sulfonamide, 5-chloro. Product catalogs toss in registry numbers (for example, CAS 66135-36-8) and supplier codes, but the core identity runs true to the ring, sulfonamide at position two, and chlorine perched at position five.
Chemists never treat sulfonamides with casual disregard. While 5-Chlorothiophene-2-Sulfonamide doesn't top hazard charts, appropriate lab attire—gloves, goggles, solid shoes—stays mandatory. The molecule doesn’t vaporize easily, but dust inhalation brings respiratory hazards. Material Safety Data Sheets advise adequate ventilation or dust masks, especially at scale. Spills meet clean-up through damp cloths, never dry brushing, and all waste routes through licensed chemical disposal streams. Regulatory oversight pulls from REACH and local standards, matching global supply chain scrutiny. Eye-wash stations and chemical spill kits form a non-negotiable backdrop in operational spaces.
Industries with a foot in pharmaceuticals, agricultural chemistry, and certain advanced materials turn to 5-Chlorothiophene-2-Sulfonamide not as a final product but as a launching pad. Its structure tames unwanted reactivity and directs functionalization, useful in medicinal chemistry research for constructing specific enzyme inhibitors or in the search for anti-infective scaffolds. Crop scientists use close analogs in early-stage screens against plant pathogens. Polymer chemists find the sulfonamide group good for modulating solubility or introducing points of crosslinking in specialty materials. Never the headline act, always in orbit around vital innovation work.
Peer-reviewed journals over recent decades log an expanding set of studies exploiting the regiochemistry of 5-Chlorothiophene-2-Sulfonamide for targeted synthesis campaigns. Whether the problem asks for quick access to other thiophene-sulfonamide hybrids, or for a bit of creativity from medicinal chemists drawn to unusual scaffolds, this molecule slides readily into the experimental spotlight. The presence of both an electron-withdrawing chlorine and a handle for further cross-linking lets teams chase leads in rational drug design and probe pathways of resistance, especially as antibiotic failure rates start to climb worldwide. These practical wins in the research bench often echo in slightly tweaked forms in industry, powering innovation without the usual delays linked to regulatory re-evaluation, since the backbone stays well-characterized.
Safety studies, while sparse compared to mainstream pharmaceuticals, suggest modest acute toxicity. Structurally related sulfonamides can trigger allergic responses or skin reactions in a subset of the population familiar with antibiotic hypersensitivity. Chronic exposure data still runs thin—no surprise given the molecule’s spot as a specialty intermediate rather than a therapeutic. But prudent companies test for cytotoxicity and environmental degradation, especially as downstream products head toward mass deployment in medical or agri-tech settings. The general lesson from the sulfonamide family remains clear: well-run risk assessments save trouble before green-lighting anything beyond the bench scale.
Cheminformatics points to a quiet but steady growth curve for 5-Chlorothiophene-2-Sulfonamide and its analogs. Medicinal chemistry’s hunger for new ring systems with controlled reactivity keeps this old standard in circulation. As automation, machine learning, and high-throughput screening take deeper root, intermediates that handle both structural novelty and predictable reactivity will keep shining. Environmental and health regulations keep narrowing the field for hazardous reagents, so stable, multi-functional intermediates like this will find more homes in both small biotech start-ups and huge multinational plants. From a chemist’s viewpoint, 5-Chlorothiophene-2-Sulfonamide serves as a quietly reliable partner—never flashier than gold, but far harder to replace than headlines would suggest.
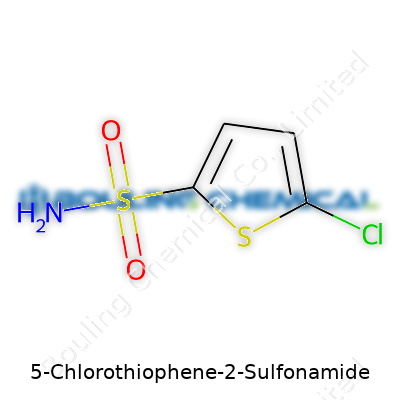
5-Chlorothiophene-2-sulfonamide packs a lot of punch in its small framework. Imagine a thiophene ring as a five-sided structure, with four carbon atoms and a sulfur atom holding hands in a circle. Throw a chlorine atom at the fifth carbon—right across from the starting line—then tack on a sulfonamide group just next to the sulfur. That’s the core of this molecule. Chemists usually sketch it as C4H2ClNO2S2. The logic behind these attachments lies in both electronic behavior and what the molecule intends to do in the real world.
Ring systems like thiophene have always drawn attention because of how they balance stability with reactivity. Chlorine at the 5-position nudges the electron cloud and can actually help direct reactions toward that sulfonamide spot at the 2-position. In drug development, these little tweaks shape the molecule’s attitude—how it interacts with enzymes, or how tough it holds out against metabolic breakdown. In labs I’ve worked in, the introduction of a sulfonamide on a heterocycle like this usually signals plans for antibacterial research. You spot these motifs in everything from industrial additives to starting points for more complex compounds.
Lab results rarely lead straight to the marketplace, because researchers can’t ignore the health and safety aspects. Placement of that chlorine atom changes more than just a line on a paper—it can reduce the reactivity at one corner and make the molecule stickier or harder to metabolize. Many times, altering positions or substituents flips the entire game from a possible medicine to something less desirable. Schering and Pfizer built blockbuster drugs around these sorts of templates, but every change required a mountain of safety checks. There’s a reason responsible chemical design always comes back to core structure.
Chlorinated thiophenes usually show tricky behavior. Sometimes they give off odors, irritate skin, or linger in the environment. Disposal becomes a headache. Years ago, our team had to rethink protocols because a structurally similar compound hung around in wastewater and showed up where it shouldn’t. Here’s where the lesson comes home: solid knowledge of how each functional group impacts environmental fate shapes responsible decisions. That means not just understanding the molecule, but also staying on top of scientific literature and following stronger disposal protocols. Regular audits and peer reviews in the lab keep teams honest and accountable.
There’s no shortcut. Success comes from relentless research, honest self-assessment, and biting the bullet on production costs to support cleaner processes. Open data from academia and industry helps the entire field grow smarter. Investing in green chemistry—like designing molecules that break down more easily or swapping out more persistent groups—addresses root problems. Lab coworkers and I have spent more late nights than I care to count chasing after unexpected byproducts, but the drive comes from making sure new structures like 5-chlorothiophene-2-sulfonamide can serve their purpose without creating bigger headaches for future generations. Every atom matters.
5-Chlorothiophene-2-sulfonamide sounds like a tongue-twister, but in a chemistry lab, it tells quite a story. This compound pulls together a thiophene ring, chlorine, and a sulfonamide group into one small molecule. Those three features give chemists a lot to work with, especially in drug design and agrochemistry. Throughout my years in science, I’ve encountered variants of thiophene endlessly because they offer both stability and reactivity, setting up useful reactions that can go way beyond textbook theory.
Sulfonamides opened the door to modern antibiotics almost a century ago. Pharmaceutical labs don’t let go of a reliable motif easily, and 5-chlorothiophene-2-sulfonamide lands squarely in many medicinal chemistry pipelines. It often serves as a core to craft new drug candidates, particularly targeting enzymes where sulfonamides already showed promise. Chlorinated thiophenes can sneak into biological systems in specific ways, binding to proteins and altering their functions. This compound gets plugged into research around diuretics, anti-inflammatory drugs, and cancer treatments. Teams often report that swapping a hydrogen for chlorine on the thiophene ring tweaks the whole drug’s behavior—sometimes making it more effective, sometimes tamping down unwanted side effects.
Farmers and horticulturalists rely on more than just sweat and sunshine—they need modern crop protection. Chemists draw from chlorothiophenes and sulfonamides to create new herbicides, fungicides, and pesticides. 5-chlorothiophene-2-sulfonamide serves as a scaffold for molecules that can fight plant pathogens and weeds. I’ve heard researchers swap out the functional groups on this compound to test hundreds of combinations, sifting for candidates that kill off a fungus but leave crops untouched. Selectivity matters, and compounds based on this structure sometimes provide it when older chemicals fail.
Not every scientist using this compound is making drugs or weed killers. Some need it for what it represents—a flexible chunk of aromatic chemistry with a couple of highly reactive spots. In a research lab, having a sulfonamide on a thiophene ring can open a pathway to more complex molecules that are otherwise tricky to access. During my time in research, these sorts of motifs have become go-to starting points for those wanting to test brand-new reactions. That flexibility can speed up discovery, shave off steps in making complex targets, or just bring new tools into reach for synthesizing dyes, materials, or small-molecule probes.
Every time a new chemical goes into a lab or gets close to crops or pets, questions around safety pop up. The properties of sulfonamides and thiophenes draw scrutiny—regulators and academics push for more toxicity data and environmental impact reports. Responsible labs submit plenty of paperwork, run countless assays, and check how long a compound hangs around in the soil or water. I’ve watched researchers lose funding or face setbacks when toxicology results crop up late in the game. Even the best new compound doesn’t go anywhere without a strong record of both lab and environmental safety.
The world keeps asking for smarter medicines, tougher crops, and cleaner chemistry. Compounds like 5-chlorothiophene-2-sulfonamide fuel these advances. The trick lies in making the best use of each molecule’s strengths while keeping human health and environmental stewardship in focus. Labs that balance innovation and safety help move science—and society—a little further down the road.
Chemical discussions can make eyes glaze over, but sometimes the details matter more in real life than in the lab. Take 5-Chlorothiophene-2-sulfonamide for example. This molecule features a blend of sulfur, chlorine, nitrogen, and aromatic carbon rings packed into a single compound. The molecular formula comes out as C4H4ClNO2S2. Chemists and pharmacists run into this compound because it offers a mix of interesting chemical behaviors, mostly due to the sulfonamide group sitting on a reactive thiophene ring with a chlorine atom attached at the number five position. The formula isn't just a string of letters and numbers for paperwork. It tells you what to expect about how this molecule acts in test tubes and, if developed, out in the real world.
Taking the atomic weights and adding them up, the molecular weight lands at 213.67 g/mol. This number pops up on labels, in lab notebooks, and in calculations, because dosage, reactions, and purity checks all depend on knowing it exactly. I remember triple-checking molecular weights as a student, partly from fear of screwing up entire experiments, partly because one miscalculated dose could throw months of work into the trash. With 5-Chlorothiophene-2-sulfonamide, even a small error in weighing results in losses—chemicals aren't cheap, and cross-contaminating glassware with a compound of this size means everything gets washed, dried, and checked all over again.
When you look at compounds like this, there’s a story behind the arrangement. Sulfonamide groups pop up all over medicinal chemistry. Starting in the 1930s, sulfa drugs changed healthcare, bringing modern antibiotics into the picture. Today, chemists hunt for molecules that combine biological activity with unique properties, and adding sulfonamide to an aromatic ring like thiophene helps with that. Chlorine ups the game, adding possibilities for reactivity or, in some cases, selectivity towards certain targets in the body. Researchers see potential in these structures for building new pharmaceuticals, dyes, or agrochemicals. Molecules like this serve as stepping stones, not just final products, in synthesis routes. Every substitution or tweak can dramatically shift both safety and effectiveness.
I’ve seen up close how a tweak—like adding a chlorine atom—might turn a bland molecule into something with promising biological activity. Yet, this also brings new safety concerns. Chlorinated and sulfonated compounds often require extra care in handling and disposal. The same features that increase biological potency can raise environmental hurdles. Watching colleagues wear double gloves and work behind shields wasn’t because they liked the look; it comes down to real safety issues stemming from chemicals just like this.
Development never sits still. As labs dive deeper into sulfur- and nitrogen-based compounds, there’s always a need for rigorous oversight. I’ve noticed the pattern: a breakthrough gets attention, companies want to ramp up production, but environmental and health safety officers double down on reviews. Any chemist planning to make or use 5-Chlorothiophene-2-sulfonamide can’t just focus on the product; waste management and workplace safety sit on equal footing. These compounds bring promise, but they also test our responsibility. The formula and weight may seem dry, but in the right hands, they support discovery and demand respect.
Getting storage right for compounds such as 5-Chlorothiophene-2-Sulfonamide matters more than many realize. Every lab professional who’s watched a research plan break down over degraded stock appreciates the hassle. This substance, which finds its place in pharmaceutical and chemical research, responds just like other sulfonamides—stable under some conditions, but not so forgiving once things turn sloppy.
Contamination and moisture destroy the purity of many sensitive reagents. Years spent working in different research settings have shown that even a little humidity exposure quickly makes sulfonamides clump and turn unreliable. Store 5-Chlorothiophene-2-Sulfonamide in an airtight container. Go for glass over plastic, since some plastics leach chemicals over time, and they flex with temperature changes. Place that container in a cool spot, ideally between 2°C and 8°C—regular refrigeration or a climate-controlled room work well.
Direct sunlight spells trouble. Ultraviolet light often breaks down sensitive chemicals, even when containers look opaque. Wondering about light protection often gets dismissed, but degradation sneaks in faster than many expect, even in partial shade. Shield samples in dark jars or wrap clear glass in foil. A drawer or a closed cabinet in the fridge adds one more layer of protection, keeping the structure intact longer.
Sulfonamides remain pretty stable under dry, cold settings, but moisture is the enemy. Water molecules react with this sulfonamide over time, opening the ring structure and ruining batch consistency. A little contamination from repeated opening and closing sets off a chain of issues. Even if the chemical looks fine, impurity levels climb. Always use a sterile spatula—never scoop with bare hands or contaminated tools. Minimize time out of storage by prepping workstations and planning ahead.
Air exposure means oxidation. Even a brief moment on a humid day, combined with oxygen, triggers slow chemical change. Desiccators filled with silica gel can stave this off, especially if your workspace sits in a damp climate zone.
In my own lab work, unopened sulfonamides like this one, properly sealed and stored, typically keep their activity for at least two years, sometimes stretching to three with rigorous care. Opened containers, especially those exposed regularly, fall off quickly in consistency before the end of that period. Loss in potency or purity doesn’t always show up visually or through routine smell checks.
Quality suppliers often assign a two-year shelf life to unopened bottles—based on stability studies, not just tradition. If you push past that date, expect results to drift. Analytical labs should schedule periodic re-testing for older stocks, using techniques like HPLC or NMR to check for decomposition or impurity buildup. Small-scale users should buy only what they’ll use within a year, avoiding bulk discounts unless the compound stays sealed for most of that time.
Lab safety manuals sometimes get ignored in busy work, but small steps keep chemicals like 5-Chlorothiophene-2-Sulfonamide solid for ongoing projects. Good training, careful housekeeping, and clear labeling make the difference between trustworthy results and weeks of troubleshooting. Choose smaller containers if possible, rotate stock, and keep detailed records of when each bottle sees first use. These habits, picked up over years of benchwork, cut down on wasted supplies and save researchers from repeating experiments due to subtle batch failure. Solid science starts with tight control over every compound’s home and history.
Chemists and lab workers cross paths with a mountain of compounds, and 5-chlorothiophene-2-sulfonamide pops up in research or process development. Skipping over its safety data just to save minutes has led to injuries in labs. Years back, a friend ended up with chemical burns from an organic compound—he thought it seemed harmless, but a splash landed on his bare wrist and taught him never to cut corners. Chemical names like 5-chlorothiophene-2-sulfonamide often go unnoticed until something goes wrong.
This compound doesn’t get much mainstream attention, but its structure resembles some known irritants and sulfonamides. The halo-thiophene and sulfonamide groups are worth a pause. Halogenated organics sometimes trigger allergic reactions or skin troubles. Sulfonamide fragments have a reputation—they’ve caused allergies ever since the early days of antibiotic use. MSDS sheets for similar chemicals list warnings: irritation to skin, eyes, or lungs on contact. A splash or accidental spill can cause harm, especially in a bustling lab or an industrial environment with lots of moving parts.
I’ve seen students wipe their faces after handling similar powders. A rash, stinging sensation, and eye redness quickly followed. This sort of discomfort is well-documented with many sulfonamide compounds. All it takes is a split-second oversight when transferring samples, and hands become conduits for trouble. Fans blowing, hands moving, and the next thing you know, dust drifts out. Some dust forms are easily inhalable—another entry point for risk.
Gloves seem basic, but people often overlook the need for face shields and goggles. Several safety alerts in academic labs encourage using chemical fume hoods because vapors and dust can linger. It’s the tiny particles that sneak up—even a mask won’t always protect if the hood isn’t running right. Beyond PPE, I always check if a chemical could react with others nearby, especially acids or strong bases, since reactions can release gases or heat on contact.
Accidental spills happen, no matter how careful people claim to be. Fast action matters: dousing the affected area with water, removing contaminated clothing, reporting the spill, and using spill kits for powders. No heroics—just well-trained reflexes. I’ve learned that storing sulfonamide derivatives away from oxidizers or heat sources keeps everyone safer. Labeling and secondary containment make a difference. Once, a poorly sealed jar leaked crystals onto a bench. The cleanup chewed up half a day, and all because someone overlooked sealing the container properly.
Many hazards only show up after years of handling a compound. For 5-chlorothiophene-2-sulfonamide, the published data isn’t as robust as for solvents or acids, but similar chemicals warn us. Chronic exposure can lead to skin sensitization or respiratory allergenic effects. The way forward isn’t fancy: invest in proper ventilation, keep up with safety training, and foster a culture where reporting concerns isn’t met with eye rolls.
Putting in the work—reading the new MSDS sheets, doing walk-throughs, and asking colleagues about their experiences—should become routine. There is no replacement for actual vigilance and respect for chemicals, even those that seem unremarkable at first glance.
| Names | |
| Preferred IUPAC name | 5-chlorothiophene-2-sulfonamide |
| Other names |
5-Chloro-2-thiophenesulfonamide 5-Chlorothiophene-2-sulphonamide |
| Pronunciation | /ˌfaɪˌklɔːrəˌθaɪ.əˈfiːnˌtuːˌsʌl.fəˌnæm.aɪd/ |
| Identifiers | |
| CAS Number | 'CAS Number: 42963-91-7' |
| 3D model (JSmol) | `4f2w5e25e85e3e85d5e6d5e25e4b6156317a` |
| Beilstein Reference | 1202760 |
| ChEBI | CHEBI:28498 |
| ChEMBL | CHEMBL416191 |
| ChemSpider | 21569387 |
| DrugBank | DB07715 |
| ECHA InfoCard | 03c3c5e4-318d-46e2-a519-0dfd92754800 |
| EC Number | 7426-35-9 |
| Gmelin Reference | 96952 |
| KEGG | C14375 |
| MeSH | D017980 |
| PubChem CID | 20898190 |
| RTECS number | GF8575000 |
| UNII | RC8O6UO0G1 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | 6G7YA7J264 |
| Properties | |
| Chemical formula | C4H4ClNO2S2 |
| Molar mass | 177.63 g/mol |
| Appearance | White to Off-White Solid |
| Odor | Odorless |
| Density | 1.63 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | 0.19 |
| Vapor pressure | 0.000253 mmHg at 25°C |
| Acidity (pKa) | 6.46 |
| Basicity (pKb) | pKb = 11.08 |
| Magnetic susceptibility (χ) | -63.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.6500 |
| Dipole moment | 3.09 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 365.3 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H302 + H315 + H319 + H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P302+P352, P305+P351+P338, P312, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | > 185.0 °C |
| NIOSH | GV5950000 |
| PEL (Permissible) | Not Established |
| REL (Recommended) | 10 mg/mL |
| Related compounds | |
| Related compounds |
Thiophene-2-sulfonamide 5-Bromothiophene-2-sulfonamide 5-Chlorothiophene 2-Chlorothiophene 5-Chlorothiophene-2-sulfonic acid 5-Methylthiophene-2-sulfonamide 5-Chloro-2-thiophenecarboxamide |