Chemistry never stands still. Over the years, folks chasing new possibilities have turned their eyes to heterocyclic compounds. 5-Chlorothiophene-2-Carboxylic Acid became a target in the 20th century as scientists saw the potential of thiophene rings in everything from drug discovery to material science. Early synthesis routes used harsh halogenations, later swapped for less wasteful methods. The uptick in medicinal chemistry research in the 1970s and 1980s really pushed demand, driving labs to refine and scale up processes. Each stage of development responded to actual hurdles: scarcity of starting materials, poor yields, tricky purifications. Experienced chemists didn’t shy away from getting their hands dirty, running adjustments batch after batch, learning which solvents or temperatures gave cleaner products or fewer byproducts. The progress happened because real people logged each failure and success, rather than just chasing the next big thing.
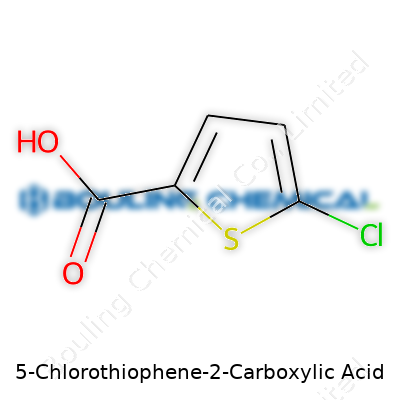
Look at 5-Chlorothiophene-2-Carboxylic Acid and you see a white or slightly yellowish powder—nothing flashy at first sight. The molecular structure unites a thiophene ring with a carboxylic acid at the 2-position and a chlorine hanging on the 5-position. It’s the backbone for many syntheses but rarely the end point. In academic settings and fine chemical manufacturing, it’s often stocked in glass bottles, dry and protected from light to keep air and moisture from spoiling the batch. Folks in the industry know that tiny changes in shipment or storage mess with performance down the line. That’s why lots of companies have protocols for handling, labeling, and tracking product integrity throughout its shelf life.
The real hands-on experience with this compound starts when you’re looking at melting point and solubility. Expect a melting point in the ballpark of 130-135°C. In my own experience, moisture creeps in easily if you’re not vigilant, pushing the melting point down and messing with purities. The acid function increases polarity, allowing decent solubility in polar organic solvents like methanol or ethanol, though it usually resists dissolving in pure water unless you adjust pH. Chlorine at the 5-position makes for distinct reactivity, giving it more edge compared to unsubstituted thiophene acids. Solid at room temp, you won’t see strong odors, but you shouldn’t trust your nose for identification—check on a real instrument instead.
Quality control introduces clear benchmarks for assay purity, typical in the range of 98% or higher. Color, particle size, and loss on drying appear on most spec sheets, reflecting how customers actually use the compound in R&D or scaled syntheses. Labeling covers major safety cues, but having worked in both a lab and on shipping docks, I’ve seen the confusion that sets in when containers aren’t marked with lot numbers and expiration dates. This traceability supports later root-cause analysis if anything goes sideways in a synthetic run. Tech data sheets focus on melting point, NMR spectra, and potential contaminants, which set the bar for downstream reliability. If trace metals or solvents stray above limits, whole batches of downstream chemicals could land in the waste bin—real-world implications, not just numbers on a certificate.
Manufacturers usually start with thiophene-2-carboxylic acid, hitting it with controlled chlorination. People who just read a procedure don’t see the grind it takes to get decent yields. Old methods tried using elemental chlorine, but that approach creates a jungle of byproducts. Today’s preferred methods rely on milder chlorinating agents like NCS (N-chlorosuccinimide), which grant a higher selectivity for the 5-position while cutting down on hazardous waste. Solvent choices like acetic acid or DMF come from trial and error—every batch reminded us that a cleaner reaction cuts work hours at the purification stage. Crystallizing the final acid often turns into a puzzle, where small tweaks in temperature or solvent ratio mean the difference between sharp white crystals and a sticky mess.
Chemists prize this compound for convenience in modifications. The chlorine holds up in cross-coupling reactions, letting you build more complex heterocycles via Suzuki or Stille conditions. In practice, a robust supply of boronic acids or stannanes can give you a library of new thiophene-based molecules. That carboxylic group doesn’t just sit idle, either. You can turn it into esters or amides, giving entry points for bioactive compound synthesis or polymer projects. People rarely keep the structure unchanged; most efforts go toward swapping groups or linking to larger frameworks. Time in the lab teaches that keeping your glassware scrupulously clean and adding reagents slowly matter as much as picking the textbook conditions.
Plenty of naming systems can trip up even experienced chemists. 5-Chloro-2-thiophenecarboxylic acid equals 5-CTCA in shorthand. U.S. suppliers list it under the CAS registry number 24065-33-6. Overseas distributors sometimes call it 2-carboxy-5-chlorothiophene. Cross-checking synonyms matters. Without tight controls on naming, someone ordering for research could wind up with a close cousin that throws off a big project. In my time sourcing chemicals for different university departments, miscommunication over names ended up wasting both money and precious synthesis time.
Safety training makes the real difference here. 5-Chlorothiophene-2-Carboxylic Acid doesn’t explode or ignite like some lab regulars, but it can irritate the skin, eyes, and airways. Standard PPE—gloves, goggles, and lab coat—keeps risks low, but only if people follow through every single time. Good ventilation matters, especially during weighing or transferring to avoid dust. On the shipping and storage end, labeling containers with hazard statements from GHS ensures everyone down the line knows what they’re handling, not just the original user. Disposal should never go straight down the drain; collecting waste through designated channels protects both workers and the wider ecosystem. All these standards result from past mistakes—every line on a safety data sheet ties back to someone learning the hard way.
Researchers and companies use 5-Chlorothiophene-2-Carboxylic Acid because it offers versatility. Medicinal chemists rely on it for making anti-inflammatory and anti-microbial candidates. Agrochemical companies test derivatives as building blocks for plant-protection agents. The electronics industry uses certain thiophene derivatives in organic semiconductors and conductive polymers. Each application pushes users to tune the reaction conditions more carefully and monitor purity, as even tiny impurities or structural changes influence results and product performance. In the pharmaceutical sector, this acid helps generate core skeletons for drugs in the preclinical development phase. That work connects directly to improving health outcomes when successful molecules transition out of the lab.
Nobody in R&D likes plateaus. Labs keep pushing the scope of how this molecule might fit into new drug scaffolds or next-generation materials. Recent research leans into green chemistry, finding less polluting chlorination strategies or inventing process intensification steps using microflow reactors. Structure-activity relationship studies (SAR) draw on 5-chlorothiophene-2-carboxylic acid as a launchpad, sometimes leading to surprising new activities in bioassay screens. Scalability remains a big challenge. When scaling up from a few grams to kilos or beyond, thermal profiles and mixing don’t simply scale linearly—watching batch runs for hot spots or crystallization nightmares proves that real scaling demands both theory and hands-on adjustments. Professional pride comes from not just making something new, but making it better, cheaper, and less harmful.
Guidelines push for more data on toxicity. Acute oral and dermal tests in animal models place the compound in a category that doesn’t trigger intense restrictions, but that doesn’t mean it deserves less respect. Chronic exposure, environmental breakdown, and metabolite tracking represent areas with limited published data—regulatory changes in REACH and global harmonization create constant pressure to update dossiers. Labs that care about long-term reputation invest in third-party studies, not just because of legal requirements but to actually manage risks. For university environments, accidental spills or releases highlight the need for quick containment and proper reporting. Keeping up-to-date on regulatory shifts means fewer surprises during audits and safer products for everyone downstream.
Looking at future demand, two powerful trends stand out: push for greener syntheses and development of high-value applications. More academic groups and startups are optimizing methods for less hazardous waste and lower energy use. Meanwhile, advances in organic electronics and targeted pharmaceuticals promise to grow the need for custom thiophene acids. Synthetic biologists and material scientists are already experimenting with ways to insert this core motif into biosynthetic pathways or designer coatings. I’ve seen many teams in early-stage development hunting for commodity molecules that deliver consistency and potential for modification—the kind of platform chemical this acid represents. Progress will come from listening to feedback out of the lab and along the supply chain, keeping focus on real-world needs over theoretical possibilities.
Every chemical name tells a story. 5-Chlorothiophene-2-Carboxylic Acid sounds complicated, but breaking it up simplifies things. The core structure, thiophene, is a five-membered ring holding four carbons and a sulfur atom. The “5-chloro” means there’s a chlorine atom on the fifth position of the ring. “2-carboxylic acid” means a carboxyl group attaches on the second position. Combine these features, you get a clear image: this isn’t just a random assembly of atoms, but a scaffold that researchers and chemists rely on. The chemical formula for this compound is C5H3ClO2S.
Sitting in a classroom or scrolling through online databases, it’s easy to gloss over why accuracy is so crucial. But in the lab, a missing atom changes how a substance behaves. Every subscript and letter in a chemical formula affects its properties, interactions, and safety. I’ve worked on projects where one tiny slip in structure meant lost weeks of effort and lots of troubleshooting. For people in pharmaceuticals or agrochemicals, getting a formula wrong could become a much bigger problem—costly errors or even dangerous batches.
5-Chlorothiophene-2-Carboxylic Acid crops up in several synthesis routes. Chemists reach for it when building new molecules, especially for testing as possible drug components or crop treatments. This only works if the starting formula holds up. Even academic researchers need to trust that C5H3ClO2S stands for exactly what it’s meant to. Otherwise, results lose meaning, and reproducibility flies out the window.
The impact of one wrong digit shows up far outside small research labs, too. For example, chemical suppliers print this formula on their bottles and make those details public for compliance and safety sheets. Regulatory agencies—like the FDA or EPA—often audit these records. Accuracy trickles down to the people mixing solutions onsite, the scientists checking purity, and the warehouse staff storing it safely.
It's tempting to lean on Wikipedia or crowd-sourced sources for chemical formulas, but peer-reviewed chemical databases like PubChem, Sigma-Aldrich, or ChemSpider exist to reduce human error. These databases list C5H3ClO2S as the formula for 5-Chlorothiophene-2-Carboxylic Acid, backed by experimental references and global registry services (such as CAS numbers).
Double-checking details in certified sources before ordering or using a chemical catches mistakes before they snowball. Quality control departments play a huge part. In my own experience, cross-referencing suppliers’ certificates with trusted database entries prevented shipment delays and confusion during audits. Software tools that auto-validate structures against IUPAC standards also clear up many issues before compounds hit the workbench.
The correct formula, C5H3ClO2S, does more than sit on a label; it serves as a trust anchor for professionals who depend on it for health, safety, and innovation. This foundation supports research, discovery, and the products that influence daily lives. Paying attention to detail, using reputable resources, and pushing for strong training in scientific literacy keep everything on track—and keep people safe.
5-Chlorothiophene-2-carboxylic acid often flies under the radar, quietly anchoring work in pharmaceuticals, crop science, and advanced materials. In practical terms, this molecule’s significance starts with its core structure: a thiophene ring with a chlorine atom and a carboxylic acid group. Chemistry classrooms rarely mention it, but walk past the research bench in any synthetic chemistry lab, and it’ll pop up on the bottle labels.
The pharmaceutical industry leans on 5-chlorothiophene-2-carboxylic acid as a starting block for drug creation. Medicinal chemists value the thiophene ring because it brings stability and the chance to tweak molecular properties. Researchers link this compound to other fragments, creating prototypes for medicines that target infections, inflammation, or neurological issues. Companies patent new molecules each year that list this acid as the key building material. Without solid, reliable starting materials, drug pipelines dry up, and real-world treatments never make it past lab notebooks.
This approach echoes stories shared by colleagues in drug chemistry. Years ago, a friend spent months grafting compounds onto thiophene-based frameworks, hunting for a new antibiotic. The carboxylic acid group made modification possible. Only by using these versatile acids did the discovery project reach animal testing.
Farmers face shifting weather and tough pests. Agricultural scientists use 5-chlorothiophene-2-carboxylic acid to create new pesticides and herbicides. This compound doesn’t show up in the field itself but gets transformed into products that boost food yields and reduce crop loss. It matters because fewer crop failures keep food prices steady at the store. Even small advances in pesticide chemistry ripple through global supply chains, feeding more people and easing the strain on arable land.
Electronics and sensor research also depend on unusual molecules. 5-Chlorothiophene-2-carboxylic acid adds functional diversity when designing conductive polymers or advanced nanomaterials. Lab teams modify the molecule’s structure to shape the way electricity flows through films and coatings. Such tweaks help invent thinner, smarter displays and more sensitive sensors — the kind that measure pollution or track health markers. It’s easy to overlook these technical details, but performance leaps often start at the molecular level, with craftspeople shaping the smallest building blocks into futuristic products.
The chemical sector always faces questions about safety, waste, and environmental impact. Factories must process chlorinated compounds like 5-chlorothiophene-2-carboxylic acid carefully. Outdated methods can miss toxic byproducts or produce waste that’s hard to treat.
Switching to greener synthesis methods has become practical. Flow chemistry and microwave-assisted techniques use less energy and cut down on hazardous solvents. Newer protocols favor atom economy, making sure each ingredient gets used up with little waste. Regulatory agencies keep a close watch, pushing the shift further each year. Taking chemical stewardship seriously protects lab workers and surrounding communities, a lesson many teams have learned firsthand after scares and near-misses.
5-chlorothiophene-2-carboxylic acid will keep pulling its weight for years to come. Its value lies in how it helps build useful things: medicines, crop protection agents, and advanced electronics. Choosing cleaner production routes and keeping a close eye on waste will create even more reliable supply chains and safer work environments. That kind of progress reaches from the chemist’s glovebox to the dinner table and the hospital ward.
5-Chlorothiophene-2-Carboxylic Acid has carved out its spot as a key intermediate in agrochemical and pharmaceutical research. Every chemist hoping to run a semi-decent reaction knows this much: if the material isn’t pure, the entire batch can turn into a headache. Most suppliers list purity on the spec sheet, and it usually lands between 97% and 99%. For a smaller molecule, that sounds right. So, what’s the real story behind those numbers?
Anyone who’s cleaned up a botched synthesis or had a subtle impurity ruin a spectrum knows that “close enough” sometimes misses the mark. The big challenge with 5-Chlorothiophene-2-Carboxylic Acid rests in its impurity profile. Halogenated heterocycles like this one tend to attract trace by-products—chlorinated, oxidized, or even polymeric bits that sneak through rough purification routines. Those leftovers do more than pad out the mass; they can shift melting points, throw off NMR readings, and tank reaction yields.
Regulatory bodies such as the FDA and EMA have zero patience for hidden contaminants. If you’re in drug development, a batch with 98% assay on paper might ship red flags if it carries even trace genotoxic residues, or it throws inconsistent bioactivity in screens. That’s not some distant regulator’s concern—it’s real, local lab frustration. A friend working at a generic API facility used to tell me how a single percent of leftover thiophene dimer could foul up HPLC so badly they’d lose days finding the culprit.
The best commercial batches of 5-Chlorothiophene-2-Carboxylic Acid show up at 98% purity, measured by HPLC or GC. Mass spec can tease out those minor peaks, but not every vendor offers those details. Recrystallization and flash chromatography can crank the number higher—but not without cost. If you twist a supplier’s arm for 99%+ purity, the price tag grows. Some research-only companies offer 99% or higher, but large-scale batches usually stick under 99%. It’s rare to find a kilogram drum at “analytical grade” unless you’re paying extra or ordering a custom lot.
Why pay the premium for those last couple of percent? Reliable reactions. For published research, reproducibility depends on well-defined starting points. I remember a medicinal chemist who spent weeks troubleshooting a coupling reaction, only to discover the real issue: just shy of 1% unknown halogenated by-product at the start. Once he swapped to a fresh, cleaner batch, yields soared. Cheap becomes expensive when your whole downstream process derails.
Certificates of analysis (COAs) help, but one always needs to sample check. Trusted vendors share their full panel—water content, melting point, maybe a chromatogram. Shady sources might sell “technical grade” product with specs that match on paper, but in practice, show wide inconsistency. For regulated industries, batch-to-batch reliability matters as much as high purity. Even small changes in impurity levels can throw off scale-up.
If high purity is mission-critical, run your own QC check on incoming material before dumping it into your glassware. Use multiple techniques: HPLC for profile, NMR for structure, Karl Fischer for water, and keep GC handy for volatiles. Work with vendors who offer transparency, not just a number at the bottom of a page.
In the end, purity isn’t just a number—it’s reliability, safety, and data you can trust. You get what you confirm, not just what you pay for.
If you handle chemicals in a lab or industry, there’s no room for shortcuts. 5-Chlorothiophene-2-Carboxylic Acid asks for that same respect. Its safety data sheets explain the potential harm in clear terms. Those who have spent time in chemical storerooms know the importance of living up to those warnings. Even a routine day turns into a hazardous one with simple neglect—a spill, a reaction, a forgotten open cap. Proper storage provides the baseline for safety, not only for the product but for everyone nearby.
I have learned that temperature always matters, not just for stability but for peace of mind. Keep this compound away from direct sunlight and store it in a cool, dry area. Warm storage rooms lead to chemical degradation, moisture, and potential volatility over time. Reliable, climate-controlled cabinets, set below normal room temperature, slow down any breakdown. A chemical like this dislikes fluctuating conditions. If water somehow finds its way inside, even trace moisture can start unwanted reactions or degrade product quality. Desiccant packs go a long way in keeping bottles bone dry.
Anyone who’s poured a chemical into a poorly chosen bottle knows glass isn’t always just glass. Solo storage on a sturdy shelf, inside an amber or opaque glass bottle—this keeps reactive light away. Screw-top lids seal tight, refusing humidity and air the chance to sneak in. Edged by supporting racks, bottles shouldn’t wobble or tip. If the container cracks, always use a replacement. Plastics sometimes react over the months, so chemical-resistant glass gives you an extra layer of security. Label bottles with content, date, and hazard signs, skipping shorthand and keeping the information plain for the next person. Avoid housing acids like this one near strong bases, oxidizers, or reducing agents. Mixing fumes or accidental drips can lead to severe incidents that no one wants to deal with at closing time.
Some of the worst lab accidents start with unauthorized handling. Only trained personnel should access strong acids. In labs where the work never really stops, using keyed cabinets or digital locks keeps outsiders away. Don’t tuck bottles behind others or shove them onto crowded shelves. Neat rows, sorted by chemical family and risk, cut confusion and help in a pinch. During emergency drills, I’ve seen quick access to safety sheets and a clear storage layout make all the difference. This small act supports a culture of care that spreads through a team.
Even the best labeling and placement mean little if you never check on the chemicals. Routinely inspect the containers for leaks, discoloration, or broken seals. Small issues turn into big emergencies once they’re ignored. Place a spill kit within arm’s reach of the storage area, not buried under paperwork or forgotten behind unused equipment. For seasoned lab workers, a spill isn’t just a stain on the floor. It’s exposure risk, property damage, and cleanup headaches. Everyone benefits from simple, regular shelf checks and keeping the spill kit up to date.
Solid storage practices only go so far without current records. Digital logs that mark inventory, shelf life, and disposal dates build a safety net. Before chemicals become liabilities, up-to-date logs help schedule safe removal or replenishment. Clear assignment of responsibility brings trackable accountability and invites everyone in the space to take ownership for shared safety. Consistent training ensures newcomers follow the same rules that keep old hands out of trouble. Protecting people and product value comes down to creating habits, not just rules in a binder.
5-Chlorothiophene-2-carboxylic acid tends to show up in research labs, especially in places where folks build new drugs or advanced chemicals. Looking at the name, a few red flags already pop up. Anything with “chloro” in the label points to chlorine stuck to a ring molecule. That generally reads: respect in the lab, no matter how often a chemist handles these chemicals.
The safety data sheet tells the tale. This powdered compound kicks up dust. Breathing that dust isn’t a recipe for health. Long-term exposure might irritate lungs or nasal passages. If it gets onto the skin, a burning itch might follow, and drops landing in the eyes send folks scrambling for the wash station. Lab assistants and seasoned chemists I know keep gloves and goggles close. Spills on bare hands never get shrugged off.
The real kicker: nobody really wants to know how it tastes. Accidentally swallowing even a little could bring on nausea or abdominal pain. Folks who work with these kinds of chemicals usually steer clear of snacks or coffee on the job, since a spill on a lab bench stays on the surface until it’s fully scrubbed clean.
5-Chlorothiophene-2-carboxylic acid does not burst into flames easily, but heating it to high temperatures might send toxic vapors into the air. Most organochlorine compounds, when burned, let off harsh fumes, including nasty chlorine-based gases. I remember an incident in grad school where a poorly sealed waste container warmed up in direct sunlight, venting a cloud of strong-smelling gas that cleared the lab for hours. Fire from this stuff is more than just smoke and flames — it’s a health threat.
No chemical like this belongs near food or drink. Safety advice from years in labs always sticks: fit the gloves tight and pull on goggles before scooping even a pinch. Work under a fume hood, which pulls vapors and dust away from the breathing zone. If powder scatters, everyone grabs a HEPA vacuum or sweeps very gently to avoid clouds. I’ve seen what happens if folks skip such steps: nosebleeds, rashes, and hours waiting in the campus clinic.
Label clarity makes a mark, too. Sloppy penmanship or missing hazard symbols cost time and risk mistakes. One colleague once stored a similar organochlorine in a mislabeled jar. Weeks later, the containment crew lost an afternoon double-checking everything on the shelf because nobody could be sure what had leaked. Consistent labeling stops that headache.
Sometimes researchers push to switch out compounds like 5-chlorothiophene-2-carboxylic acid for less risky ones. Green chemistry isn’t just buzz — reducing harmful chemicals replaces messy cleanup with smoother workflows. Replacing chlorinated rings when possible makes the routine less dangerous for new lab techs or students who might miss a safety step.
Chemical waste from research doesn’t get poured down the drain at the end of the day. Most labs stash used solvents and solids into special bins for chemical disposal teams, and everyone gets regular reminders to follow strict disposal rules. One careless pour could harm pipes, groundwater, or even wildlife living nearby. Using a product safely means thinking beyond just today’s experiment — people look after each other and the planet with every step.
5-Chlorothiophene-2-carboxylic acid asks for respect. It rewards careful hands and punishes shortcuts. If people keep goggles on, label every jar, and lock up the dust, everyone goes home healthy. A bottle like this isn’t just another lab supply; it’s a reminder that safety in science never runs out of style.
| Names | |
| Preferred IUPAC name | 5-chlorothiophene-2-carboxylic acid |
| Other names |
5-Chloro-2-thiophenecarboxylic acid 5-Chlorothiophene-2-carboxylate |
| Pronunciation | /ˈfaɪ-klɔːr.oʊ.θaɪ.oʊˈfiːn tuː kɑːrˈbɒk.sɪl.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | [19690-06-9] |
| 3D model (JSmol) | `7-Oc1sccc1C(=O)O` |
| Beilstein Reference | 1101180 |
| ChEBI | CHEBI:85770 |
| ChEMBL | CHEMBL484206 |
| ChemSpider | 21819978 |
| DrugBank | DB08398 |
| ECHA InfoCard | 18e77ce5-779c-44b9-b7c4-764160204361 |
| EC Number | Not assigned |
| Gmelin Reference | 668637 |
| KEGG | C19122 |
| MeSH | D000080004 |
| PubChem CID | 69717 |
| RTECS number | XT3150000 |
| UNII | TI836PSM8I |
| UN number | Not classified |
| CompTox Dashboard (EPA) | DTXSID6063906 |
| Properties | |
| Chemical formula | C5H3ClO2S |
| Molar mass | 174.62 g/mol |
| Appearance | white to off-white solid |
| Odor | Odorless |
| Density | 1.570 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | 1.82 |
| Vapor pressure | 0.0000161 mmHg at 25°C |
| Acidity (pKa) | 3.57 |
| Basicity (pKb) | 1.96 |
| Magnetic susceptibility (χ) | -35.6 × 10^-6 cm³/mol |
| Refractive index (nD) | 1.624 |
| Dipole moment | 3.87 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 192.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –279.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1112.1 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, causes skin irritation |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364, P403+P233, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 113.7 °C |
| Lethal dose or concentration | LD50 oral rat > 5,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2000 mg/kg |
| REL (Recommended) | 40 mg/m3 |
| IDLH (Immediate danger) | There is no specific IDLH (Immediate Danger to Life or Health) value established for 5-Chlorothiophene-2-Carboxylic Acid. |
| Related compounds | |
| Related compounds |
Thiophene-2-carboxylic acid 5-Bromothiophene-2-carboxylic acid 5-Iodothiophene-2-carboxylic acid 5-Methylthiophene-2-carboxylic acid 5-Chlorothiophene 2-Chlorothiophene-5-carboxylic acid |