Chemistry never stands still, and 5-Chloro-2-(trifluoromethyl)-pyrazine has a place in that constant push for better building blocks. Back in the late 20th century, researchers began searching intensely for new pyrazine derivatives. The introduction of halogens and trifluoromethyl groups became a favorite method because these changes brought serious tweaks to the reactivity and stability of the parent structures. 5-Chloro-2-(trifluoromethyl)-pyrazine came out of that period as a specialty intermediate, earning greater attention for pharmaceutical and agrochemical synthesis. The trifluoromethyl group often turns a simple molecule into a contender in everything from crop protection to advanced therapeutics. Just looking at patent records provides a timeline — by the mid-1990s, this compound started popping up in various synthetic pathways, both in academic journals and industry filings.
5-Chloro-2-(trifluoromethyl)-pyrazine looks pretty unassuming to anyone outside chemistry: a crystalline solid, white to off-white depending on the purity and batch. The real value hides in its chemical backbone. Placing a chlorine atom at the five position and a strongly electron-withdrawing trifluoromethyl at the two position changes how this pyrazine reacts, allowing chemists to steer selectivity in many reaction types. The compound doesn't grab headlines but fits into a long lineage of fine chemicals essential for making more valuable targets. Both research labs and chemical manufacturers buy and sell this compound globally, sometimes under trade names but more often with just the basic chemical description.
5-Chloro-2-(trifluoromethyl)-pyrazine clocks in with a molecular formula of C5H2ClF3N2 and a molecular weight around 182.5. The melting point typically lands in the range of 40-44°C, solid at room temperature, and it's stable in usual storage conditions as long as the container stays dry and sealed. It dissolves well in polar organic solvents like acetonitrile and DMSO; water solubility drops off dramatically thanks to the trio of fluorines and the pyrazine ring. The compound doesn't stray from the odorless and colorless norm of many fine organic intermediates, but its high volatility means chemists need to keep containers tightly capped. With its electron-deficient ring, it often stands out in electrophilic aromatic substitution reactions, tolerating a range of milder bases and acids, unlike many more fragile heterocycles.
Suppliers express quality through a purity assay, commonly listed above 98% by GC or HPLC. Container sizes vary, but most outlets provide it in 5g, 25g, and 100g increments. Labeling must comply with GHS guidelines, owing to the chlorine and trifluoromethyl substituents that imply health hazards. Each vial demands details: lot number, molecular formula, batch purity, and risk pictograms. Handwritten notes rarely appear here. Barcode tickets and digital tracking systems attach to shipments, especially for international orders. Certificates of analysis accompany every lot, giving reference spectra, assay results, and, in some stricter locales, proof of origin to satisfy regulatory checks on chemical precursors.
Industry and academia have converged on a handful of efficient routes. The most common synthetic approach uses either 2-chloro-5-nitropyrazine or 2-(trifluoromethyl)pyrazine as starting points. Nucleophilic aromatic substitution and direct halogenation form the backbone of the lab-scale method. The process often starts with a pyrazine ring, trifluoromethylation by reaction with CF3 sources such as Ruppert-Prakash reagent, and then selective chlorination using reagents like N-chlorosuccinimide under controlled temperatures. Column chromatography or recrystallization handles purification. Larger manufacturing batches leverage continuous flow reactors for better yield and control, keeping byproduct levels low and minimizing solvent waste. Chemists keep an eye on moisture during reactions, as water can hydrolyze sensitive intermediates, eating into yields.
The search for new molecules naturally leads to a spotlight on the functional groups of this compound. The chlorine stays in play for nucleophilic displacement. This reactivity positions 5-chloro-2-(trifluoromethyl)-pyrazine as a target for Suzuki, Buchwald, or Ullmann-type couplings, with the possibility of attaching heterocycles, aryl groups, or even complex pharmaceutical fragments. The trifluoromethyl group resists most mild chemical buffers but pulls electron density out of the ring, which increases the speed and selectivity in downstream C-H activation processes. Other transformations tap into reduction and amination at the pyrazine ring, with medicinal chemists often tweaking the molecule to dial up potency or make analogues for antifungal, antibacterial, or crop protection portfolios. Derivatization can branch into nitro-reduction, amidation, or even click-chemistry if functionalization is required downstream.
This compound answers to many aliases, depending on supplier catalog or research context. Common alternative names include 5-chloro-2-trifluoromethylpyrazine, 2-(trifluoromethyl)-5-chloropyrazine, and CAS number 105270-35-1. Some specialty chemical firms attach their own codes, but standardized nomenclature rules reign for regulatory and compliance reasons. When searching research articles or patents, these synonyms help track down parallel work or commercial availability, opening up the world of chemical information to those willing to drill a bit deeper than the first search result.
People working with 5-chloro-2-(trifluoromethyl)-pyrazine face the same chemical safety landscape as with many halopyrazines. Lab SOPs generally call for nitrile gloves, splash goggles, and use of a chemical fume hood. The material can irritate skin and eyes, and inhalation causes respiratory discomfort in poorly ventilated spaces. Supplier SDS sheets underline the need for immediate decontamination with copious water in the event of accidental contact, and chemical-resistant containers avoid reactivity with vapors or solid residues. Waste management hinges on local guidelines for halogenated solvent destruction; most labs contract these duties to specialized hazardous waste handlers. Anyone scaling up reactions receives extra training, given the volatility and toxicity of the byproducts sometimes formed in these processes.
Few molecules get pigeonholed forever, and 5-chloro-2-(trifluoromethyl)-pyrazine proves that point. Its main value cascades from use as a synthetic intermediate, laying the groundwork for more intricate compounds that end up in pharmaceuticals, pesticides, and specialty fine chemicals. Medicinal chemists grab it as a stepping stone for anti-infective drug candidates, using those electron-withdrawing groups to build out structure-activity relationships. Agrochemical researchers slot it into crop protection projects, betting on the combination of halogen and fluorine atoms to confer environmental stability and target pest selectivity. Material chemists sometimes screen derivatives for new liquid crystal or electronic properties, aiming at display or sensor applications, though that niche remains smaller. This compound brings flexibility to anyone who needs to tune a core heterocycle, and its continued use proves that even subtle structural tweaks pay off in chemical discovery programs.
Researchers continue probing new routes for greener, faster syntheses, with a big push toward milder reagents that trim hazardous waste and energy bills. Automated reaction screening and AI-guided retrosynthesis are now in the toolkits of many labs, speeding up the hunt for next-generation functionalized pyrazines. Drug discovery teams model analogs on computer clusters, predicting which substitutions could boost efficacy or cut toxicity. Most open-access chemistry databases show a steady stream of new derivatives, especially in structure-activity screens for antimicrobial, antitumor, and insecticidal properties. Companies invest in application notes and process patents to protect their edge, but academic publications continue driving this area forward with open data on yields, byproducts, and reaction mechanisms. This compound, like many others with halogen and trifluoromethyl tags, signals an intersection between tradition and innovation in small-molecule R&D.
No chemical gets into widespread use without toxicological scrutiny, especially in pharmaceutical and agricultural supply chains. Early readouts on 5-chloro-2-(trifluoromethyl)-pyrazine did not show acute toxicity at levels comparable to other halopyrazine building blocks, but full profiles require looking at every route: inhalation, dermal, oral, and environmental fate. Sometimes the byproducts or metabolites create more concern than the parent compound itself, so regulatory agencies push for exhaustive impurity profiling. Cytochrome assays, substrate-enzyme binding studies, and chronic exposure screens continue to fill in the picture. The trifluoromethyl group carries environmental persistence, which means any release outside controlled facilities calls for monitoring and reporting. Ongoing research tracks the fate of these molecules in water and soil, as long-term effects can slip past standard acute exposure panels.
Many chemists see a bright future for 5-chloro-2-(trifluoromethyl)-pyrazine, provided safety and waste issues don’t overwhelm the practical advantages. The continued importance of the pyrazine ring in drug and agrochemical discovery keeps this compound in regular production, and greener synthesis using catalysis or biotransformation stands to clean up older routes. As industries move toward more sustainable pipelines, demand will shift to manufacturing routes that trim resource consumption and minimize persistent waste. Big leaps in machine learning-driven molecule design forecast a new wave of pyrazine scaffolds, and 5-chloro-2-(trifluoromethyl)-pyrazine is well placed as both a building block and research reference. If regulatory guidance continues to tighten around halogenated compounds, new safety data and alternative replacements could shape future demand or drive targeted innovation in ring substitution patterns. Overall, careful stewardship of this compound’s production and handling sets the pace for its role across multiple research and industrial domains.
In the world of chemistry, names often sound complicated, yet every piece of a compound’s name reveals something tangible. For 5-Chloro-2-(Trifluoromethyl)-Pyrazine, the formula spells out its identity: C5H2ClF3N2. Looking at these letters and numbers, there’s a story about how chemists build molecules for medicine, agriculture, and beyond.
Pyrazine rings belong to a class of aromatic heterocycles — the backbone for many pharmaceuticals and agrochemicals. Toss in a trifluoromethyl group and a chlorine atom, and the molecule shifts in function and behavior. That trifluoromethyl group at position two means three tightly bound fluorines replace what could have been hydrogens on the ring, making the molecule more resilient against breakdown. The chlorine at position five tweaks both the electronic structure and how the molecule interacts with other substances.
For folks outside the lab, it’s easy to look at chemical formulas and tune out. But think about medical research: a tiny substitution or addition—a few atoms here or there—can mean the difference between an effective drug and an inert powder. The formula C5H2ClF3N2 is not just information for chemists; it underpins the way researchers design experiments and build new products. In pharmaceutical development, adding a trifluoromethyl group sometimes boosts how well a compound gets absorbed in the body, or how long it sticks around to do its job. The same logic applies in crop protection, where a small group on a pyrazine ring helps a treatment stick through a rainstorm or block pests for a whole growing season.
Working with fluorinated compounds like this one calls for respect. Fluorine atoms raise the heat stability and metabolic resistance of molecules, but disposal and environmental impact stay front of mind. Compounds with chlorine and fluorine don’t break down as easily in water or soil. That durability means more persistent residues in ecosystems and food chains. Regulators in Europe, the U.S., and Asia release guidelines all the time based on new toxicology or persistence data. Companies can’t afford to cut corners: transparent sourcing, traceability, and proper waste handling keep workers and communities safe. Labs that use C5H2ClF3N2 invest in rigorous effluent controls and keep detailed records. Mistakes, even small ones, turn headlines in moments, and for good reason—long-lived chemicals shape the world long after a shipment leaves the plant.
No one solves chemical challenges alone. Academic chemists, pharmaceutical companies, and regulators have started to move toward greener approaches. Safer synthetic methods, new catalysts, and biodegradable alternatives find their way into the industry pipeline every year. Some firms tweak the pyrazine core, swapping chlorine out for less persistent groups, or choosing alternatives to heavy halogenation. Others improve analytical testing so residues show up at vanishingly low levels. Real progress means listening to environmental scientists, factory workers, and end users—the people who bear the burden when companies cut costs or overlook long-term hazards.
Chemistry remains about choices. Each extra fluorine or chlorine is a decision with downstream effects. C5H2ClF3N2 offers benefits, but the conversation keeps growing: can chemists design with both performance and sustainability in mind? In my own time working hands-on with halogenated compounds, I saw the complexity firsthand: balancing risk, cost, performance, and impact takes honesty and care at every step. Every chemical formula hides more than numbers; it holds a blueprint for how science, industry, and society can move forward together—or fall behind.
5-Chloro-2-(Trifluoromethyl)-Pyrazine gets a lot of attention in labs where new pesticides and herbicides come to life. In my time collaborating with agricultural researchers, I’ve seen how this molecule finds its way into the backbone of popular crop protection agents. Many herbicides rely on the pyrazine ring for their sharp activity against invasive weeds, and the trifluoromethyl group makes the whole structure more robust by improving resistance to the sun and rain. Chemists use this compound as a core building block, leveraging its halogenated structure to tune bioactivity, which translates to crops with better yields and fewer headaches for farmers.
Pharmaceutical chemists look for molecular frameworks that push the boundaries of what’s treatable and how long medicines stay active in the body. Over the past decade, I’ve spotted 5-Chloro-2-(Trifluoromethyl)-Pyrazine pop up throughout patent filings from major drug developers. Its combination of chlorine and trifluoromethyl shapes the molecule’s behavior inside the body, often boosting absorption or stalling metabolic breakdown. There is documented evidence in research journals where this pyrazine helps form the base skeleton for anti-infectives, anti-cancer drug programs, and central nervous system agents. For instance, the molecule serves as a precursor in certain kinase inhibitors, designed to block harmful overgrowth of cells.
In discovery chemistry, flexibility counts. This compound shines as a starting point for architects of new materials. Synthetic chemists appreciate its ability to undergo various reactions—Suzuki couplings, nucleophilic substitutions, among others—pushing the creation of more intricate molecules with precise electronic and physical properties. In an advanced chemistry lab I visited, researchers combined it with boronic acids to craft molecules targeting unique protein structures. The fluorinated, chlorinated pattern on the pyrazine ring alters reactivity, opening up unique reaction channels not seen with simpler analogs.
Researchers in electronics and specialty materials need chemicals that can survive high heat, UV light, and aggressive solvents. By introducing 5-Chloro-2-(Trifluoromethyl)-Pyrazine into polymer backbones or coatings, material scientists boost resistance and lengthen product lifespans. I’ve discussed with colleagues in coatings businesses who shared how such pyrazine derivatives help produce finishes for machinery that shrug off corrosion or constant wear. The molecule’s profile—halogenated, fluorinated—plays a role in resisting harsh conditions, a key requirement for building better insulators and protective films.
Every industry using sophisticated building blocks like this faces a challenge: maintaining safety and embracing sustainability. I’ve seen first-hand how responsible handling in the lab can limit exposure and waste, but pressure mounts industry-wide for greener substitutes or recycling processes. Chemists investigate milder reaction conditions, waste-minimizing protocols, and safer alternatives where possible. Investment in closed systems and improved personal protective equipment can prevent accidents. My own experience echoes how direct, ongoing dialogue between suppliers, regulators, and users supports safer usage, helping the world benefit from 5-Chloro-2-(Trifluoromethyl)-Pyrazine without unnecessary health or environmental risks.
Walking into any chemistry lab, purity levels draw interest right away. I’ve seen researchers chase after that elusive 98% mark for compounds like 5-Chloro-2-(Trifluoromethyl)-Pyrazine. From the plant floors of contract manufacturers to the focused atmosphere of pharmaceutical R&D, I know folks care about purity because a few decimals make a visible difference to results. A batch clocking in at 97% often spark discussions: Is it enough for the next stage, or will those small impurities send projects spinning out?
From what suppliers and chemical listings show, most commercial 5-Chloro-2-(Trifluoromethyl)-Pyrazine lands between 96% and 99% purity. Better grades bump toward 99% and get specifically offered for pharmaceutical synthesis. Analytical certificates and HPLC chromatograms, provided by any responsible seller, spell out just how clean the material looks. I’ve learned to always request a certificate of analysis up front. Cutting corners on this piece of paper leads to headaches later, whether stuck with byproducts contaminating a pathway or odd results needing endless troubleshooting.
It’s tempting to shrug off the gap between 96% and 99%, but impurities rarely behave. I watched colleagues lose weeks tracing back unexpected HPLC peaks, only to find traces from the reagent—which then required a new batch, introducing delays and extra costs. Routine compounds like 5-Chloro-2-(Trifluoromethyl)-Pyrazine sometimes look simple, but non-target molecules creep in through unreacted starting material, side-reactions, or poor storage. Even 2–3% unknowns shake up final product yields or trigger harsh regulatory scrutiny, especially in drug development.
For synthetic chemists, those “trace” contaminants now often mean retesting, more purification, or even changing the supplier. Downstream, active pharmaceutical ingredient (API) manufacturers rely on high-purity feedstocks to avoid regulatory penalties and batch failures. The fact that the FDA and EMA recommend impurity levels below 0.1% in finished APIs illustrates how the chain of purity builds on itself.
Demand for ultra-clean reagents forced chemical producers to step up, and I find more suppliers these days investing in better fractionation, gas scrubbing, and checking against calibrated reference materials. Sourcing direct from a supplier with a record of consistency beats fishing for bargain prices. Quality teams know the pain of having to qualify a new vendor just because a previous order showed out-of-spec purity.
It helps to create a checklist: ask up front for batch-specific chromatograms, request residual solvent data, and pay attention to long-term storage recommendations. Taking these steps upfront saves plenty of grief later. If the application involves making a lead compound destined for animal studies or clinical phases, even more scrutiny falls on every input chemical.
Lab mistakes usually trace back to skipped checks, not faulty theory. In my own experience, I trust brands only after comparing their certification data with independent analysis. I ask analytical chemists to run a quick NMR or GC-MS scan just for confirmation. That extra half hour avoids costly mishaps. Five minutes spent reading the data sheet beats five days repeating a failed synthesis.
Taking time to verify the quality of 5-Chloro-2-(Trifluoromethyl)-Pyrazine keeps science on track. Purity isn’t just a figure—it decides which experiments succeed, which batch ships, and which results stand up to peer review.
Experience shapes the way I see lab safety, especially with chemicals that pack a punch. 5-Chloro-2-(Trifluoromethyl)-Pyrazine, like many substituted pyrazines, demands respect. This compound offers unique features as a building block for pharmaceuticals and agrochemicals, but it can put staff and capital investments at risk without careful storage and handling. A single missed step might expose people, equipment, or product quality to hazards. Guidance on best practices doesn’t just tick the box for compliance — it protects real people and investments that keep research afloat.
Every lab worth its salt keeps chemicals like 5-Chloro-2-(Trifluoromethyl)-Pyrazine away from heat, sunlight, and moisture. Shelves or cabinets built from non-reactive materials improve safety. Strong ventilation protects against inhalation risks in case a spill or container breach happens. I’ve seen companies value clear labeling, both on containers and shelves, so technicians don’t fumble in a rush or during audits.
Quality control teams use sealed, airtight containers made of glass or high-grade plastic. This stops volatile fumes from seeping. Dedicated chemical refrigerators extend shelf life, controlling temperature spikes and humidity. In my own weekly rounds, expired or partially used chemicals never stay on shelves. Disposal happens fast—old stock becomes a risk, not an asset.
Segregation proves essential for compounds like this. Reactive chemicals, especially acids or strong bases, stay in their own zones. The same goes for organics and oxidizers. Accidental cross-contamination between incompatible classes leads to runaway reactions, and nobody wants to mop up a mess, or worse, rush for a shower station. Regular inventory checks keep chemicals accounted for and out of desperate hands.
You can’t cheat proper personal protection. Lab coats, nitrile gloves, and tight-fitting safety glasses form the front line. My knuckles still remember a careless splash from a misjudged pour years ago—those burns heal, but lesson sticks. Fume hoods aren’t for show. They provide a safe way to transfer, weigh, or divide chemicals like this. Sensitive noses can tell when someone cuts corners.
Standard procedures call for no eating, drinking, or touching your face near 5-Chloro-2-(Trifluoromethyl)-Pyrazine. Washing hands afterwards, even if gloves stayed on, stops residue from hitching a ride home. In real practice, spill containment kits should stand ready within arm’s reach. Absorbent materials, neutralizing agents, and waste containers keep an “oops” from turning into a disaster. I’ve seen labs turn spill drills into friendly competition—fast reflexes and clear heads under stress don’t come from one-off training.
Record-keeping takes on renewed importance. Every movement, use, and disposal deserves a spot in the logbook. Auditors and safety officers appreciate transparency, and in the rare case of a recall or incident, a good paper trail saves days of headache.
It’s no secret that 5-Chloro-2-(Trifluoromethyl)-Pyrazine can cause skin and eye irritation, not to mention respiratory issues, especially if mishandled. Proper signage remains a feature, not a suggestion. I encourage making spill response and first-aid protocols common knowledge—everyone remembers the steps because lives might hang on them. Clear communication and steady supply of PPE support a safety-first culture every day.
Waste streams demand tight control. Used containers, pipettes, and gloves go into hazardous waste bins, not the regular trash. Reliable disposal partners with records of compliance—these can’t be afterthoughts if a lab wants to stay out of trouble.
Continuous training, scheduled inspections, and active involvement from everyone keep mistakes from turning into tragedies. People and process together keep compounds like 5-Chloro-2-(Trifluoromethyl)-Pyrazine working for the greater good, not as sources of harm.
Digging into information about chemicals such as 5-Chloro-2-(Trifluoromethyl)-Pyrazine, it’s easy to find a list of suppliers or hints about possible uses in pharma or agriculture. The real struggle begins with trying to answer a simple question: just how safe is this molecule to work with? And what do we really know about its toxicity? The moment you check those usual sources like PubChem or the ECHA database, you discover that reliable, published data on both short-term and long-term exposure takes some real effort to find.
Lack of safety data leads to guesswork, and there’s a lot at stake. Many labs and production facilities work under tight regulations for good reason. If you don’t know whether a compound causes skin irritation or respiratory issues, you have two choices: treat everything with maximum caution, or risk making a bad call. That costs time, money, and—if someone gets hurt—the consequences can last much longer than a single shift.
From my years in the field, most chemists don’t want to work in the dark. The reality is, sometimes a chemical enters the workflow because it fills a need in synthesis, and then everyone scrambles to do the background safety checks. For a substance like 5-Chloro-2-(Trifluoromethyl)-Pyrazine, I've had to dig deep into supplier SDS sheets just to get a hint, only to find “data not available” under acute toxicity, reproductive effects, or carcinogenicity.
Without solid studies, we start making guesses based on chemical relatives. Chlorinated and fluorinated pyrazines often don’t show up in household products. They usually belong to the toolbox of those building new molecules, not the ones making things for the public. But this doesn’t mean they’re harmless. Chlorine and trifluoromethyl groups both have reputations: chlorine atoms on aromatic rings may linger in the environment, and highly fluorinated groups can show bioaccumulation and resist breaking down.
No amount of experience replaces actual testing. A missing LD50 or data gap in environmental fate drives a wedge through planning safe waste disposal or deciding protective measures for workers. The case of perfluorinated substances should ring a bell for anyone following the news about persistent organic pollutants.
Getting real answers means pushing for more transparent research and publishing both positive and negative toxicity findings—not just the green lights that let a substance slide onto the market. Public chemical databases need this detail, and so do industry safety teams. In many places, regulatory rules demand at least a minimal hazard assessment before anything goes from lab to market, but loopholes let some chemicals slip through with pretty thin paperwork.
Pressure needs to stay on for better disclosure and stricter rules around manufacturing and importing poorly studied chemicals. Small companies, startups, and academic labs often lack resources to fund full studies, and that’s where public-private partnerships or grant programs come in. Open-access toxicology data serves everyone: researchers, workers, and even the families who rely on people coming home safe.
With 5-Chloro-2-(Trifluoromethyl)-Pyrazine, until there’s a clear safety and toxicity record, I treat it like an unknown. Gloves, fume hoods, and closed waste streams become the rule, not the exception. Curious minds will keep asking the hard safety questions. That protects people and the reputation of responsible labs everywhere. Facts—easy to check, tough to find—shouldn’t be a luxury.
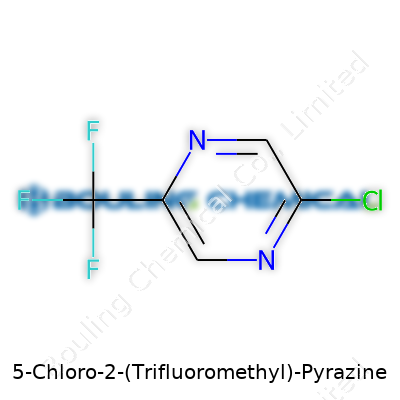
| Names | |
| Preferred IUPAC name | 5-chloro-2-(trifluoromethyl)pyrazine |
| Other names |
5-Chloro-2-(trifluoromethyl)pyrazine 5-Chloro-2-trifluoromethylpyrazine 2-Trifluoromethyl-5-chloropyrazine 5-chloro-2-(trifluoromethyl)pyrazine |
| Pronunciation | /faɪˈklɔːrəˌtuːˌtraɪfluːəˈmɛθəl.paɪˈreɪziːn/ |
| Identifiers | |
| CAS Number | [32843-07-9] |
| 3D model (JSmol) | `3Dmol('CC1=NC=C(N=C1)C(F)(F)F')` |
| Beilstein Reference | 1433662 |
| ChEBI | CHEBI:189897 |
| ChEMBL | CHEMBL3211109 |
| ChemSpider | 20206677 |
| DrugBank | DB08343 |
| ECHA InfoCard | 03-2119-007273 |
| EC Number | NA |
| Gmelin Reference | Gm. 827056 |
| KEGG | C19475 |
| MeSH | D014452 |
| PubChem CID | 294883 |
| RTECS number | XU4025000 |
| UNII | HS27RZH2VT |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID2020779 |
| Properties | |
| Chemical formula | C5H2ClF3N2 |
| Molar mass | 178.55 g/mol |
| Appearance | Light yellow liquid |
| Odor | Odorless |
| Density | 1.53 g/cm3 |
| Solubility in water | Insoluble |
| log P | 2.7 |
| Vapor pressure | 0.2 mmHg (25°C) |
| Acidity (pKa) | pKa = 1.42 |
| Basicity (pKb) | “pKb = 9.99” |
| Magnetic susceptibility (χ) | -64.7×10^-6 cm^3/mol |
| Refractive index (nD) | 1.4850 |
| Dipole moment | 3.98 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 272.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -786 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P332+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-2-0 Health:1 Flammability:2 Instability:0 |
| Flash point | Flash point: 94 °C |
| Lethal dose or concentration | LD50 Oral Rat 1800 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 336 mg/kg |
| NIOSH | GT1225000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 5-Chloro-2-(Trifluoromethyl)-Pyrazine is not specifically established by OSHA or other major agencies. |
| REL (Recommended) | 10 mg/m3 |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
2-Chloro-5-(trifluoromethyl)pyrazine 2,3-Dichloro-5-(trifluoromethyl)pyrazine 2-Bromo-5-(trifluoromethyl)pyrazine 5-(Trifluoromethyl)pyrazine-2-carbonitrile 2-Chloro-5-methylpyrazine |