The imidazole ring, an old friend to chemists since the late 19th century, changed the world of drug discovery and chemical synthesis. Scientists tinkered with the basic imidazole structure, trying different substitutions, and one of the results became 5-Chloro-1-Methyl-4-Nitroimidazole. Early research focused on tweaking electron density and solubility to unlock new possibilities for pharmaceuticals and agrochemicals. Chlorination and nitration methods gathered steam as labs explored stronger, more selective reactions through the 1960s and 1970s. Growing demand for robust intermediates encouraged broader research. Over the decades, creative minds recognized the power in that combination of methyl, nitro, and chloro on the ring. The compound started turning up in patents and academic papers, sometimes as a key intermediate, sometimes as a building block for complex molecular scaffolds.
5-Chloro-1-Methyl-4-Nitroimidazole plays a supporting role in many synthetic routes, especially in pharmaceuticals and crop protection agents. Chemists see versatility in that nitro-imidazole core. By fine-tuning positions on the ring, this molecule can contribute to selective toxicity, enhance metabolic stability, and improve aqueous handling. Some routes use it to anchor side chains that increase drug-target binding. Its reliability as a starting material draws researchers and commercial chemists alike, who value solid yields and predictable behavior in large-scale reactions. Supply chains for this compound serve both bulk and specialty sectors, stretching from academic research to industrial-scale manufacturing.
This compound takes form as a pale yellow solid, typically crystallizing with sharp melting points near 130–137°C. The molecule carries a chlorinated, nitro-bearing imidazole core, and that means significant polarity, moderate water solubility, and easy compatibility with most common organic solvents. The free methyl group on the nitrogen increases stability against acidic and basic hydrolysis. With its low vapor pressure and moderate thermal stability, the material works well in batch and flow synthesis setups. Its nitro substituent draws electron density, making the ring less reactive in certain conditions, but opening the door for selective reduction without undesired side reactions.
5-Chloro-1-Methyl-4-Nitroimidazole carries the CAS Number 6968-10-7. Commercial samples arrive labeled with purity, batch number, date of manufacture, recommended storage temperature, and hazard warnings for irritancy and environmental impact. High-grade material offers purity above 98% by HPLC. Moisture content often stays below 0.5% by weight to ensure reliable reactivity. Analytical profiles from FTIR, NMR, and LC-MS support product identification and traceability. Packaging ranges from amber glass bottles to sealed aluminum drums, with appropriate hazard and handling stickers to remind users of the need for PPE and fume hood precautions.
Large-scale production tends to start with 1-methylimidazole, which undergoes selective chlorination at the 5-position, often under controlled temperature and solvent conditions using agents like N-chlorosuccinimide. From there, nitrating agents such as mixed acid transform the 4-position into the nitro derivative. Careful quenching and extraction yield the target compound with a minimum of side reactions. Some producers have optimized routes involving protection-deprotection cycles to improve regioselectivity, leading to better yields and easier purification. Process engineers monitor reaction kinetics, solvent choice, and residual byproducts to make sure the final compound meets strict purity criteria for regulated industries.
The most useful part about this molecule comes from its ready reactivity at the nitro and chloro positions. Reductive chemistry can take the nitro group down to an amine, setting the stage for all sorts of follow-up chemistry — think ureas, amides, or even heterocyclic expansions. The chloro site works as a handle for nucleophilic substitution, so users can swap in everything from simple alcohols to complex thiol-based linkers. Researchers have explored catalyst-driven cross-couplings with boronic acids, bringing unusual aryl or vinyl groups into the imidazole ring. Methyl-1 substitution resists unwanted N-dealkylation, so the molecule stands up well to tough synthesis conditions. This stability also gives process engineers more freedom to design multistep synthesis plans around it.
This compound wears several hats, depending on the context. Synonyms include 5-chloro-1-methyl-4-nitro-1H-imidazole, 4-nitro-5-chloro-1-methylimidazole, and 4-Nitro-5-chloro-1-methylimidazole. In industry catalogues, it sometimes travels under shorthand as CMNI or CNI or simply “chloronitromethylimidazole.” These alternative names streamline inventory searches but can confuse buyers if label checks fall through. Registries like PubChem and ChemSpider help sort out these variations with unique identifiers, linking back to the base chemistry and ensuring accurate sourcing.
Workers and researchers dealing with 5-Chloro-1-Methyl-4-Nitroimidazole keep safety data sheets close at hand. Prolonged exposure can irritate eyes, skin, and airways. The nitro group flags concerns about skin absorption and potential mutagenicity, so gloves, goggles, and fume extraction remain part of standard operating procedures. Regulations classify this material as hazardous, requiring clear labeling and documentation for storage, handling, transport, and disposal. Drum storage stays below room temperature and out of sunlight to slow decomposition, especially on long shelves. Trusted suppliers deliver detailed COAs with test results on batch impurities and advice for emergency spill management.
Medicinal chemistry draws heavily on this intermediate while working toward antiparasitic, antibacterial, and antifungal drugs. Nitroimidazole derivatives have shaped the backbone of treatments for conditions like giardiasis, trichomoniasis, and anaerobic bacterial infections. The presence of chloro and nitro substitutions tweaks biological uptake and cell membrane crossing, turning this scaffold useful for designing new active pharmaceutical ingredients, especially where drug resistance runs high. Agrochemical companies also build on this skeleton, exploring new classes of crop protection agents targeting fungal blights and insect pests. More recently, materials scientists have looked to imidazoles for conductivity and photostability, so 5-Chloro-1-Methyl-4-Nitroimidazole sometimes serves in functional polymers or specialty coatings.
Academic labs use this compound as a launching pad for structure-activity relationship (SAR) studies. Chemists synthesize wide arrays of analogs, testing subtle modifications for gains in biological activity. By swapping in different nucleophiles or reducing agents, researchers sketch out the limits of the imidazole’s medicinal use, mapping metabolism in vitro and in vivo. Industry partners focus on scaling up the chemistry, improving yields with continuous flow, greener solvents, or enzymatic catalysis. Open-access databases register every new derivative, fueling computer-aided design and high-throughput screening to chase after more potent, less toxic medicines with a low cost of synthesis. Major pharma pipelines habitually turn back to the nitroimidazole core whenever new parasitic threats or bacterial resistance headlines emerge.
Toxicologists have pored over the nitroimidazole class, tracking mutagenicity, cytotoxicity, and environmental breakdown products. The nitro group makes vigilance crucial, since some metabolic byproducts show up in genotoxic screens. After metabolic reduction in humans or test animals, the compounds may bind to DNA or proteins—this activity prompts strict regulatory scrutiny. In lab animals, acute toxicity varies widely with dose and route of exposure, but chronic exposure studies raise flags for developmental and reproductive toxicity if misused. That’s why manufacturers enforce worker safety and downstream users monitor bloodwork during clinical trials. Environmental impact studies look at water solubility and resistance to biodegradation, as traces might slip into wastewater. Data drive risk management approaches, encouraging best practices for waste treatment and site monitoring.
Looking ahead, next-generation research promises tailored modifications to the imidazole ring. Drug designers chase new frontiers in antimicrobial therapy, especially for infections dodging traditional antibiotics. Smart chemoinformatics digs deeper into analogs with altered ring electronics, swapping out the nitro group or tweaking the substitution pattern to sidestep toxicity while keeping strong antimicrobial action. Environmental chemists pursue degradation-friendly analogs that break down faster or with less toxic byproducts. On the industrial side, continuous process improvement seeks greener chemistry—less waste, milder conditions, and full solvent recovery. Advances in microwave and photochemical synthesis offer shorter routes and higher atom economy. 5-Chloro-1-Methyl-4-Nitroimidazole stands well at the crossroads of scalable chemistry, urgent healthcare needs, and environmental responsibility. Creativity, vigilance, and strong teamwork across labs hold the key to unlocking its next big leap in value.
Walk into any drugstore and you’ll spot shelves full of remedies for stomach bugs and infections. But behind many of those everyday products, chemists work with a cast of unsung chemical characters. One such compound is 5-Chloro-1-Methyl-4-Nitroimidazole. Not a household name, but plenty of doctors and pharmaceutical engineers know it well.
Metronidazole changed the game for fighting certain parasites and bacteria years ago. It worked against the nastier bugs like Giardia, Trichomonas vaginalis, and even some stubborn anaerobic bacteria. 5-Chloro-1-Methyl-4-Nitroimidazole falls in the same club. Chemists tinker with imidazole rings, adding things like chlorines or nitro groups, hoping to boost how strongly the drug performs or dodge pesky resistance.
This particular version steps up as a chemical building block or intermediate. Some manufacturers rely on 5-Chloro-1-Methyl-4-Nitroimidazole to synthesize medicines that fight hard-to-kill bugs. Researchers have been exploring its effectiveness against bacteria that thrive where oxygen is low, like certain wound infections or intra-abdominal abscesses.
As someone who has paid attention to recalls in the news, quality in the supply chain worries me. If a starting material doesn't meet the bar, everything it touches downstream gets contaminated. Reputable pharmaceutical makers put extra emphasis on sourcing pure intermediates like this one. Testing for impurities, tracing batches, and transparency in synthesis set apart companies that care about safety.
Antibiotic resistance is no longer some future concern. It’s a problem hospitals see up close every day. Over years, overusing certain medicines opens the door for new drug-resistant strains. By tweaking compounds at the molecular level, researchers hope to outsmart bacteria—a kind of scientific arms race. Even small modifications like a chlorine at the right spot may revive medicines that no longer work as well.
Surveillance, patient education, and investment in new combinations or alternatives can give us more time. I’ve seen too many patients respond poorly to standard drugs. The folks in labs who produce these lesser-known intermediates help keep pipelines running for the next generation of treatments.
Chemicals like this need strict oversight. Skirting rules or cutting corners with toxic byproducts hurts more than just the bottom line. Communities near factories bear the brunt if pollution control is weak. Global regulation needs teeth, with governments holding both importers and exporters accountable. Traceability, environmental care, and transparency aren’t just checkboxes—they’re key to trustworthy medicine.
Keeping ahead of diseases calls for smart chemistry. Whether tweaking old drugs or creating new ones, compounds like 5-Chloro-1-Methyl-4-Nitroimidazole matter. Anyone who’s watched someone suffer through an infection that won’t quit understands why this field draws such focus. Behind the big brand names and flashy advertisements, these substances help shape the reality of safer, more effective care.
5-Chloro-1-Methyl-4-Nitroimidazole lands on the bench with a reputation. It’s not the scariest compound you’ll meet, but getting careless around it isn’t an option. Over the years, I’ve noticed that accidents almost always come down to two things: overconfidence and distraction. So, even with something that doesn’t make the top-ten chart of hazardous chemicals, respect still matters.
Handling nitroimidazoles means gloves, lab coats, and splash goggles play a part in daily routines. The nitro group raises a red flag—compounds with these structures sometimes irritate the skin and eyes. Rubber or nitrile gloves help keep unwanted rashes or burns away. If a spill splashes into your eye, remember water beats pride—flush immediately for at least 15 minutes and call for help. Even experienced chemists make mistakes. I’ve seen peers reach for a dropper without checking for cracks, only to watch liquid dribble onto bare hands. Lesson learned—double check before you pour.
Powdered chemicals fly around if you get heavy-handed. 5-Chloro-1-Methyl-4-Nitroimidazole isn’t known for wild volatility, but dust inhalation can cause trouble. Chronic exposure has been linked to respiratory irritation in similar compounds. Work in a fume hood when measuring or transferring powders. Respirators can be overkill for the odd gram, but if you scale up, break them out. A lab mate once pointed out trace contamination on the bench after a rushed transfer. Wiping benches regularly keeps that kind of mistake from making its way from your hands to your sandwich at lunch.
Keep this compound tightly capped, stored in a cool, dry spot, away from sunlight and sources of heat. Nitro groups sometimes encourage compounds to break down, especially under the harsh light or next to oxidizers. Store separately from acids, strong bases, or anything else with reactivity history. Label everything. That sharpie scrawl could save an intern a world of confusion. I remember sorting through an unlabelled shelf in grad school—nobody wants to relive that headache.
Disposal doesn’t leave much room for shortcuts. Collect leftovers and any contaminated materials in a designated hazardous waste container—no pouring down the drain. Labs that follow EPA and local guidelines never deal with midnight calls about chemical smells near their drains. Once, a misstep in waste segregation meant our pickup got delayed, which created an unsafe backlog of materials nobody wanted to handle.
Contact with similar compounds causes eye or skin irritation, and inhaling dust can bring on coughing and dizziness. Nausea sometimes shows up after big accidental exposures. Keep safety data sheets handy—knowledge beats panic every time. If you or a coworker start to feel strange, call for medical help without worrying about looking foolish. One quick call often solves a small problem before it grows.
Watching newcomers struggle with complex labeling or proper PPE use reminds me that nothing replaces good training. I always encourage repeating safety drills, even when folks feel embarrassed. Peer checks before major procedures help everyone stay sharp. Getting everyone on the same page creates an environment where muscle memory saves lives.
Chemistry asks for curiosity, not complacency. Whether you’re running a one-off experiment or prepping kilos for a bigger job, treating 5-Chloro-1-Methyl-4-Nitroimidazole with care protects your team, your workspace, and your project.
5-Chloro-1-Methyl-4-Nitroimidazole, a name that sounds like it belongs in a sci-fi novel, holds a significant place in both laboratories and classrooms. Its chemical formula—C4H4ClN3O2—gives a sense of what you're dealing with. The molecular weight clocks in at 177.55 g/mol. These figures might seem like textbook trivia, but they play a pivotal role for any scientist or technician interested in medicinal chemistry or analytical work.
Each letter and number in a formula like C4H4ClN3O2 maps out a path for researchers. If you’ve spent time at a lab bench, you know how having these formulas handy can mean the difference between a smooth day and several hours down a rabbit hole. With this imidazole derivative, you’re dealing with chlorine, methyl, and nitro groups, making it far from a simple molecule. Each group adds its own twist, shaping how the substance acts, both on its own and mixed with others.
In my own runs through medicinal chemistry, I’ve seen how one chlorine atom can take a familiar structure and push it toward new biological properties. Diving into the details, the nitroimidazole core often links to antimicrobial or antiparasitic activities. These aren’t guesses: published studies have connected this group of chemicals to drugs that treat infections others can’t touch. Yet the fine print—the position of each atom—matters. Change an atom and you risk losing or changing the effect altogether. Companies developing better antimicrobials pay deep attention to such formulas during each stage of drug discovery.
Numbers carry their own weight, no pun intended. The molecular weight of 177.55 g/mol does more than fill an inventory spreadsheet. It helps set up basic experiments—dissolving the right amount, running chromatography, or setting up reactions. In the early days of my own lab work, I learned how a simple typo in molecular weight could upend an entire synthesis. On the clinical side, accurate dosages hinge on these calculations. People’s safety depends on exact measurements, not rough guesses.
A molecule like this doesn’t only fascinate chemists—it also poses real safety challenges. Handling compounds with nitro groups often requires extra care, as stability shifts quickly under certain conditions. Laboratories that ignore good safety protocols risk more than lost product. I still remember the caution taken during compound storage and handling, especially as nitro compounds sometimes show unexpected sensitivity to light or heat.
On the upside, there’s a window of opportunity for new research. As resistance to classic drugs rises, the search for new nitroimidazole derivatives heats up. Chemists experiment with variations—changing positions, swapping out groups—to look for higher potency and lower toxicity. These efforts need robust chemical data, public research access, and transparency around safety protocols.
Clarity about chemical identity, structure, and mass sets the groundwork for discovery and responsible use. Universities and pharmaceutical labs both win when everyone works from the same numbers and reliable information. Sharing this knowledge openly, checking work, and keeping safety front and center gives real meaning to the formulas on the page.
Some chemicals don’t tolerate mistakes. 5-Chloro-1-Methyl-4-Nitroimidazole falls into that group. The chemical draws attention for its role in pharma and research, but that usefulness can turn risky if storage steps go sideways. One odd whiff of this off-yellow powder gets people thinking about long-term health, not just the task at hand. Working in labs taught me: ignore storage best practices, and you end up paying in both safety and wasted money.
Moisture loves to ruin imidazoles. Even small leaks can spark reactions or break down the active parts, turning a valuable reagent into a mess. Practically speaking, someone who stores 5-Chloro-1-Methyl-4-Nitroimidazole in sealed glass bottles in a low-humidity cabinet dodges problems like clumping and loss of potency. Tossing a fresh silica gel pack in the bottle adds insurance against humidity spikes. Across research settings, this step beats any “good enough” habit every single time.
Direct sunlight doesn’t play nice with nitro-compounds. Strong light triggers chemical shifts, sometimes creating byproducts you don’t want mixing into your next synthesis. That’s why professionals pick solid, opaque containers—amber glass stands strong against light, and most supply warehouses deliver it for this reason.
High temps break compounds down faster. Keeping the storage space near standard room temperature—about 20-25°C—gives the longest shelf life. If a fridge is in play, chemicals stay far from food or drink: cross-contamination stories haunt too many research labs. Big temperature changes strain bottles and pop open seals, so storage means "steady as you go" more than anything else.
People sometimes skip the theory and grab chemicals without reading the labels. Limiting access to trained staff matters more than people admit. Locked cabinets or controlled rooms stop those “quick shortcut” accidents that lead to spills, inhalation, or skin exposure. For workplaces, clear rules set out how and who gets to move or open the container. A shared log or sign-out sheet keeps everyone honest and fills gaps in communication—one simple tool that stops a lot of error before it starts.
No one enjoys clearing out old or compromised chemicals, but taking a minute to date containers and run periodic checks saves headaches in the long run. Each batch comes stamped with a date, and most manufacturers mark shelf life right on the bottle. Outdated material gets trashed through proper hazardous waste procedures, not just tossed with regular garbage. Every solid operation has someone in charge of this—small details, big protection for both people and research budgets.
Over time, small steps in chemical care stack up to serious safety and cost savings. The habits that protect 5-Chloro-1-Methyl-4-Nitroimidazole—dry conditions, cool air, no sunlight, honest labeling, and limited access—carry over to many tricky compounds. My experience echoes industry case studies: shortcuts don’t hold up, and real stewardship keeps labs running safer and more productive. Chemical storage comes down to habits, not heroics—get the basics right, and the risks shrink.
Walking into any chemistry lab, you can’t miss the emphasis people place on chemical purity. In my own years working alongside researchers and manufacturers, I’ve seen how a single percentage point can change everything. 5-Chloro-1-Methyl-4-Nitroimidazole is no exception here. Its role as an intermediate in pharmaceutical and agrochemical production means the level of purity decides if it will reach medical labs, crop science labs, or end up in a different bin entirely.
This compound comes in multiple grades. For labs running routine research, technical grade may feel like the right choice—it balances price and functional performance. But for folks formulating a drug, pharmaceutical grade stands out. These higher grades come with fewer impurities, so you don’t worry about side reactions or strange results. I remember seeing what a difference this made for a colleague—an antibiotic synthesis project that kept failing on technical grade suddenly worked after they switched to a higher-purity batch. The results spoke for themselves.
Data supports this focus, too. Regulatory agencies worldwide—whether the US FDA or EMA in Europe—draw strict lines regarding what impurities are acceptable in pharmaceutical products. Contaminants in a chemical intermediate could lead to toxic byproducts or ineffective medicines if ignored. It’s not just about ticking boxes; patient health and product safety ride on every batch.
Choosing a certain grade isn't simply about science. Costs add up fast. Chemical manufacturers charge steep premiums for high-purity product, especially on a global market where demand comes from diverse sectors. I once chatted with a purchasing manager at a midsize pharmaceutical plant who described the monthly dance between budget and purity, especially after supply chain hiccups during the pandemic. They couldn’t always get their preferred grade on time and had to pause production, resulting in lost contracts and overtime for staff. The stakes run high when just a few kilograms are missing.
Trust but verify—a lesson drilled into anyone dealing with chemicals. Certified labs use methods like HPLC and mass spectrometry to check purity. On the buyer’s side, companies keep a close eye on certificates of analysis before signing off. Years ago, a small mistake—misreading a certificate—cost my team weeks in troubleshooting. Strong traceability helps. If something goes wrong, you can trace it back in seconds instead of days, and that can make all the difference in busy operations.
One solid approach involves building strong relationships with reliable suppliers. A trusted vendor streamlines quality control and shortens the gap between problem and solution. Some buyers invest in batch reservation agreements or long-term contracts to lock in supply and avoid scrambling at the last minute. Another fix is diversifying sourcing—companies look for trusted suppliers in different regions to sidestep delays caused by geopolitical tensions or shipping snags.
Education pays off, too. Teams trained in reading certificates and understanding chemical analyses catch issues before they snowball. Having spent long days in audit prep myself, I know staff knowledge is a company’s best defense.
As the demand for advanced pharmaceuticals and crop treatments grows, the story of 5-Chloro-1-Methyl-4-Nitroimidazole’s purity becomes ever more critical. Paying attention to grade selection, supplier partnerships, and in-house expertise pays off in safer and more effective results for everyone down the line.
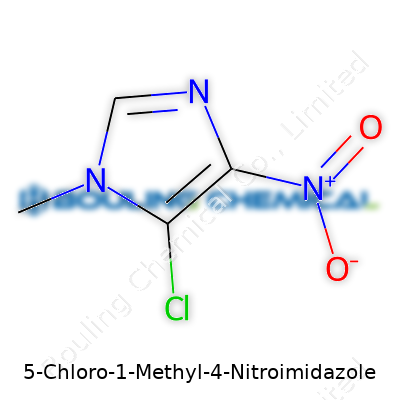
| Names | |
| Preferred IUPAC name | 5-chloro-1-methyl-4-nitro-1H-imidazole |
| Other names |
1-Methyl-5-chloro-4-nitroimidazole Clonidazole 5-Chloro-1-methyl-4-nitro-1H-imidazole |
| Pronunciation | /faɪˈklɔːroʊ waɪ ˈmɛθɪl fɔːr ˈnaɪtroʊ ɪˈmɪdəˌzoʊl/ |
| Identifiers | |
| CAS Number | 6945-17-1 |
| 3D model (JSmol) | `/3*7@@YOLeSJARffmUnDMSmEJvPaYqYZpA1RVSttK*lev^KcwjQ^TGOJMs/jp3jF` |
| Beilstein Reference | 136306 |
| ChEBI | CHEBI:48816 |
| ChEMBL | CHEMBL1561 |
| ChemSpider | 16209 |
| DrugBank | DB00916 |
| ECHA InfoCard | 03b550c4-202e-418b-97aa-890cb09e75c7 |
| Gmelin Reference | Gm. 830415 |
| KEGG | C19276 |
| MeSH | D015740 |
| PubChem CID | 18606 |
| RTECS number | TD9625000 |
| UNII | K1K3TE41XL |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C4H4ClN3O2 |
| Molar mass | 174.55 g/mol |
| Appearance | Yellow crystalline powder |
| Odor | Odorless |
| Density | 1.51 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.01 |
| Vapor pressure | 0.000022 mmHg at 25°C |
| Acidity (pKa) | 7.43 |
| Basicity (pKb) | 8.57 |
| Magnetic susceptibility (χ) | -59.56·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.555 |
| Dipole moment | 4.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 312.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -84.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1633 kJ/mol |
| Pharmacology | |
| ATC code | J01XD03 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Danger |
| Hazard statements | H301 + H315 + H319 + H335 |
| Precautionary statements | P261, P280, P301+P312, P304+P340, P305+P351+P338, P312 |
| NFPA 704 (fire diamond) | 2-2-1-OX |
| Flash point | Flash point: >110°C |
| Lethal dose or concentration | LD50 oral rat 505 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 505 mg/kg |
| NIOSH | NA9100000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | No REL established |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Metronidazole Tinidazole Secnidazole Ornidazole Dimetridazole Ronidazole |