Chemistry’s landscape has evolved on the back of small, versatile building blocks. Among the many heterocyles, imidazoles have shaped the world of pharmaceuticals, agrochemicals, and advanced materials. 5-Chloro-1-methyl-1H-imidazole did not emerge as a spotlight molecule in the classic sense, but it grew in importance during the mid-to-late twentieth century. Researchers traced its parent core, imidazole, as far back as the late 1800s, noting its widespread natural occurrence and its pivotal role in histidine and related biochemistry. As methylation and halogenation became more precise, attention turned to this particular derivative. The subtle interplay of a methyl group and a chlorine atom on the imidazole ring unlocks a unique reactivity and broadens its spectrum of application, marking a steady climb from lab curiosity to valued reagent and intermediate.
5-Chloro-1-methyl-1H-imidazole carries a special weight within organic synthesis pipelines. The molecule presents as a clear or lightly tinted liquid or crystalline solid under ambient conditions, depending on its purity and storage method. Chemists find it indispensable when constructing more complex heterocycles, as its electron-donating methyl group and electron-withdrawing chlorine offer a toolbox of possibilities for reactivity. Suppliers across Asia, Europe, and North America provide gram to ton scales, hinting at its blend of niche usefulness and industrial viability. Its catalog numbers wind through procurement databases in research and production labs, reaching medicinal chemists and materials scientists alike.
This compound’s molecular formula is C4H5ClN2. With a molecular weight of 120.55 g/mol, it blends moderate polarity with aromatic stability. Its melting point often sits just under standard room temperature, signaling its adaptable phase profile. It dissolves comfortably in polar organic solvents—think methanol, ethanol, DMF—while showing restrained solubility in water due to its hydrophobic methyl and chloro substituents. Chemists respect its air stability but keep it away from strong bases, acids, and oxidants since its imidazole ring can react or degrade under aggressive conditions. The sharp, pungent odor stands as a reminder to limit vapor exposure in confined spaces.
Proven suppliers label this compound with purity levels exceeding 97%, safeguarding reliability for sensitive applications. Product datasheets always specify batch number, percentage purity, trace metal content, spectral fingerprints (NMR, IR, MS), and recommended storage conditions—usually a cool, dry environment, protected from direct sunlight. Labels identify the chemical through both its systematic name and common synonyms, offering hazard communication in line with global GHS (Globally Harmonized System) standards, including pictograms, signal words, and risk phrases. This information empowers responsible handling across laboratories and factories.
Efficient synthesis routes often start with methylimidazole or imidazole itself as a scaffold. Direct chlorination with agents such as N-chlorosuccinimide or phosphorus oxychloride selectively introduces the chlorine atom at the desired position, often using a solvent like acetonitrile. Temperature control and reaction time demand close attention—err on the side of gentle conditions to avoid over-chlorination or ring breakdown. Post-reaction, work-up steps involve extraction, washing, and careful distillation or crystallization. Seasoned chemists track purity by thin-layer chromatography and finalize the product through vacuum drying, minimizing impurities that might compromise later stages or biological assays.
5-Chloro-1-methyl-1H-imidazole acts as more than a stop along the synthesis path. Its chloro group offers a smart handle for nucleophilic aromatic substitution. Typical transformations swap out the leaving group for amines, thiols, or even oxygen-based nucleophiles, creating a panoply of tailored derivatives. The methyl group, by its bulk and modest electron-donating effects, can also influence regioselectivity and encourage certain bond formations. Many successful routes to active pharmaceutical ingredients leverage this chemical, exploiting both its electronic and steric contributions to steer reactions cleanly toward targets.
Scientists and suppliers often cross-reference multiple aliases for the same molecule, which helps smooth communication and procurement. You may hear it called 5-chloro-1-methylimidazole, 1-methyl-5-chloroimidazole, or even leverage its registry numbers such as CAS 3436-63-9. Careful cross-checking avoids costly mix-ups. Its clarity in tracking supports reproducibility—no small matter for properly scaling up a reaction or adhering to regulatory standards on documentation and product traceability.
Handling 5-chloro-1-methyl-1H-imidazole means standing by strong chemical hygiene habits. The compound can irritate the skin, eyes, and respiratory tract—not uncommon among small, halogenated aromatics. Labs using this reagent lean into personal protective equipment: gloves, goggles, and fume hoods stand as standard fixtures. Material safety data sheets flag acute and chronic hazards, emphasizing immediate washing after contact and seeking medical attention for persistent symptoms. Proper storage—tightly closed bottles away from incompatible materials—reduces risks of leaks or cross-contamination. Waste disposal needs to follow local environmental guidelines, preventing accidental releases.
This compound’s reach cuts across disciplines. Pharmaceutical chemists tap it as a building block in crafting antifungal, antibacterial, and anticancer agents, thanks to the imidazole’s affinity for interacting with biological targets. Agrochemical developers use its reactivity to synthesize fungicides and pesticide intermediates. Beyond life sciences, it features in the formation of functional polymers, advanced coatings, and as a ligand in organometallic complexes. Each field exploits the tunable reactivity landscape provided by the dual action of the methyl and chloro groups on the aromatic core.
Academic journals and industrial patents consistently highlight new strategies to modify imidazole cores, seeking safer, greener, or more efficient transformations. Researchers have tested eco-friendly chlorinating agents and solvent-free reaction conditions, aiming to trim down hazardous waste. Medicinal chemists focus on linking the imidazole platform with novel fragments, pursuing molecules that address drug resistance or access previously undrugged targets. Computational studies help predict how the structural tweaks—like the ones on 5-chloro-1-methyl-1H-imidazole—change binding affinity in new leads. Industry R&D teams monitor these breakthroughs, adapting successful methods to scale-up operations and down the line, to large-batch manufacturing.
Toxicological profiles drive decision-making in product use and regulatory clearance. Researchers run in vitro and in vivo studies, tracking metrics such as acute inhalation toxicity, skin absorption, and potential mutagenicity. Preliminary data suggest that, like many aromatic heterocycles, the compound demands mindful handling—short-term exposure can provoke irritation, and high doses might impact neurological function or liver enzyme expression in animal models. Regulatory authorities lean into conservative exposure limits, calling for detailed risk assessments before approving applications in food-related or direct consumer products.
Developments in green chemistry open fresh routes for manufacturing and modifying this molecule. The push for sustainability prompts teams to test biocatalytic chlorination or photochemical methods that promise lower environmental impact. Growth in oncology and infectious disease medicine draws interest toward new imidazole-based scaffolds, offering fresh hope in the fight against tough pathogens or complex disease targets. Advances in catalysis and advanced materials suggest new utility for 5-chloro-1-methyl-1H-imidazole as part of functional polymers or smart surfaces. Its journey from laboratory bench to vital industrial tool continues to pick up speed, shaped by the intersection of chemical insight, safety vigilance, and creative application.
There’s a particular satisfaction in making sense of the invisible world that shapes medicines, technologies, and even flavors in our meals. 5-Chloro-1-Methyl-1H-Imidazole sounds like a mouthful, but its story is one that’s carried through laboratories and manufacturing floors worldwide. At its core, this molecule is an imidazole ring – five atoms arranged in a ring, with three of them carbon and two as nitrogen. Human curiosity has dissected these rings because small tweaks on their surface can lead to big shifts in what the compound does.
Take that basic ring, then attach a chlorine atom to the fifth position and a methyl group to the first. The methyl group carries just a single carbon and three hydrogens; it pulls electron density toward itself, making the molecule a bit less acidic and a bit more lipophilic. That chlorine at position five brings another story. Chlorine’s got heft and electronegativity, pulling charge and flirting with hydrogen bonds in all sorts of environments—be it lab glassware or inside a living cell.
Chemically, it’s written as C4H5ClN2. The skeleton looks like this: two double-bonded carbons across the ring, nitrogen atoms spaced apart at positions one and three, methyl group on one nitrogen, and chlorine swinging out from carbon five. Structural diagrams in textbooks turn this into hexagons and lines, but the real impact isn't in the drawings, it’s what these features let the compound do.
Pharmaceutical design keeps coming back to the imidazole ring system. Fungal cells, enzyme functions, and all manner of cellular switches seem to recognize imidazoles like old friends—or as threats. Swapping a hydrogen for a chlorine or dropping in a methyl group shifts the way the molecule fits into pockets in enzymes or across cell membranes. A compound like 5-Chloro-1-Methyl-1H-Imidazole has the backbone necessary for further chemical reactions, serving as a stepping stone in the creation of complex drugs or research probes.
My own experience working with scientific teams in medicinal chemistry taught me that minor changes—a chlorine here, a methyl there—turn a ho-hum chemical into something with new potential. Patents list countless derivatives where a halogen changes PK/PD profiles. Chemists keep searching for that extra bit of selectivity or metabolic stability. It's not just academic; real bells go off in production plants and pharmaceutical pipelines when a change like this hits a sweet spot.
Handling halogenated compounds brings up safety and environmental worries. Chlorine isn’t gentle if it drifts off into the wrong local waterway, and no one wants toxic byproducts piling up. Despite these drawbacks, good protocols and careful waste management push the chemical industry to keep improving. Safer storage, closed-system syntheses, and real-time environmental monitoring can prevent spills and protect workers.
From my vantage point, the value of a compound like 5-Chloro-1-Methyl-1H-Imidazole doesn’t just sit in its chemical structure. The way researchers and companies treat it—safe handling, innovative use, and environmental stewardship—translates into trust. Trust earned from regulators, from communities, and from the students now learning about five-membered rings and what happens when science dips its brush in just the right place on the molecular canvas.
5-Chloro-1-Methyl-1H-Imidazole often turns up in chemistry labs. Its value shows up in the way it builds bigger, more complex molecules. This imidazole ring, with a chlorine and methyl group, feels like a modest building block, but it has helped create everything from next-generation medicines to advanced materials. Every working chemist I know has relied on clever little molecules like this to save time and open doors in synthesis. Tossing a methyl group on the nitrogen and a chlorine on the ring gives chemists a tool that reacts differently than imidazole by itself. In drug development, that change matters. Little tweaks like these can mean the difference between a failed project and a breakthrough medicine.
The drug industry relies on small changes during early research. When a team wants to try a new direction, it feels easier to swap a known building block than to invent a brand-new route. 5-Chloro-1-Methyl-1H-Imidazole offers an easy handle for researchers aiming to make antifungal, antibacterial, and anti-inflammatory agents. The imidazole ring structure shows up in approved drugs for infections, allergies, and even cancer. Chemists swap out the chlorine for something else or use the methyl group as an anchor to attach more functional groups. This versatility opens up new possibilities, and many published papers show success using this compound in the lead-up to clinical candidates.
Industries beyond pharmaceuticals benefit too. In agrochemicals, this building block appears in syntheses of compounds that protect crops. Herbicides featuring modified imidazoles help keep food supplies stable. In the lab, adding the chlorine or methyl group tunes the molecule’s reactivity. That controls not only how the chemical interacts with pests but also how it breaks down in the soil. The goal is precise action—target the weeds, avoid harming the crops—while reducing residues that worry consumers and farmers alike.
New materials owe a lot to molecules like 5-Chloro-1-Methyl-1H-Imidazole. In electronics, some specialty polymers depend on subtle chemical features found in these rings. Electronic and optical devices—flat screen TVs, flexible lights, sensor arrays—improve when tiny functional groups tweak the properties of a plastic or thin film. Research in this area keeps racing forward, and anyone who cares about energy savings or sustainability keeps tabs on new uses for heterocycles like this.
The same features that make 5-Chloro-1-Methyl-1H-Imidazole useful can create headaches. Chlorine atoms sometimes hang around in the environment; wastewater treatment plants struggle to remove them. Widespread use in agrochemicals raises questions about runoff and persistence in groundwater. Chemists need to focus on green chemistry—using less-toxic reagents, developing safer routes, and improving ways to recycle byproducts. Tough government rules now limit emissions and waste, so every new application faces more scrutiny than ever. Cutting waste starts in the planning stage. Every chemist can design a synthetic route that reduces problematic side products and seeks safer substitutes.
The lesson here is not to give up on valuable molecules, but to treat their development as an exercise in responsibility. Incentives for greener manufacturing and cleaner waste management drive better outcomes across industries. In the classroom and in the field, conversations about sustainability need a spot on every research project checklist. Real progress builds on small choices—just like the tiny changes to an imidazole ring make all the difference for products in our homes, our hospitals, and our fields.
Handling molecules in the lab, I've seen how a slight tweak in a compound's structure changes its entire story. 5-Chloro-1-Methyl-1H-Imidazole isn’t just a mouthful—it’s a small molecule with important character. Its formula, C4H5ClN2, packs quite a bit in a small package. Folks in both academic chemistry and pharma will sometimes run into it when they want to add a twist of chlorine to the backbone of the imidazole ring system. The chlorine at position 5 and a methyl group at the nitrogen push this molecule out of “ordinary” territory.
The average chemist knows molecular weight isn’t just a number on a sheet. It shapes how a compound behaves—how it dissolves, how it gets weighed on the balance, how it handles under different solvents. For 5-Chloro-1-Methyl-1H-Imidazole, that weight lands at 132.55 g/mol. This isn’t just trivia for preparation, either. If you’ve spent hours prepping a stock solution or mapping out a reaction yield, you know just how quickly a small miscalculation on molecular weight blows everything off target. Students and pros alike check that number twice.
Take a look at 5-Chloro-1-Methyl-1H-Imidazole’s structure. The presence of both a chlorine and a methyl group on an imidazole ring means this compound wins extra attention in synthetic labs. Chlorine makes the molecule more reactive at certain positions, opening the door to new transformations. I’ve watched medicinal chemists use similar compounds as stepping stones, building more complex drug candidates starting from modest scaffolds like this.
Adding a methyl group tends to shift electronic properties, changing the rules for any reactions that follow. That small substitution affects not only how the molecule stacks or hides in biological systems, but also its solvency and volatility. As more research crews look for novel imidazole-based drugs—think antifungals or enzyme inhibitors—understanding subtle differences in weight and formula can mean fewer failed syntheses and cleaner results.
I’ve watched new lab members fumble with the difference between molecular formula and empirical formula, or misread chloride for chorine—leading to a nightmare mid-experiment. Compound authentication and verification become crucial. Unreliable sources or outdated catalog values cause confusion. Researchers open themselves to mistakes with molar solutions or stoichiometry. One practical fix: check and cross-verify the formula (C4H5ClN2) and weight (132.55 g/mol) before diving into experiments. Several accurate compound calculators exist online from trusted databases such as PubChem and ChemSpider.
In real practice, keep a mental or digital log of the compounds you use, with structures, formulas, weights, and vendors. I’ve found this habit shortens troubleshooting time and eases training for new staff. Double-checking chemical identity doesn’t just avoid waste—it ensures compliance in regulated industries and smoother publication.
Anyone handling or researching 5-Chloro-1-Methyl-1H-Imidazole knows trust in data isn’t optional. Labs have a social and legal responsibility to make sure their chemicals are what they say they are—down to the last atom. It starts with transparency about sourcing and rigorous checks using robust databases and suppliers with a record for accuracy. Reviewing safety data sheets, validating all chemical shipments, and involving experienced chemists in vetting are steps that cut back on hazards. For hundreds of compounds cycling through a building, these boring details keep both people and projects moving the right way.
Working with any chemical asks for attention and respect. 5-Chloro-1-Methyl-1H-Imidazole, like many other organic compounds, follows that same rule. It’s not about scaremongering, but about matching practical experience with what we know from research and data. I have spent years working around labs and factories, and the same basic wisdom always shows up: take care of your tools, and they’ll take care of you.
Data shows 5-Chloro-1-Methyl-1H-Imidazole irritates the skin, eyes, and respiratory tract. One quick splash and you'll wish you had worn those gloves. Repeated contact risks sensitization. Letting a compound like this float around the room or end up in your lunch is just asking for trouble. Regular use brings complacency. A proper Material Safety Data Sheet from trustworthy suppliers offers the facts with none of the fluff.
A dry, cool space goes a long way in controlling risk. Humidity tends to mess with chemical stability. Moisture creeping in can lead to slow degradation or worse, unwanted reactions. I once saw an improper cap turn a tidy storage cabinet into a sticky mess. Screw-top glass bottles work best, kept tightly sealed. Labeling becomes essential when many clear liquids look nearly identical. Storage on lower shelves prevents spillage risks. Protecting from direct sunlight keeps chemical structures intact.
Ventilation serves as the backbone of any chemical operation. Fume hoods or exhaust fans remove trace vapor before it gathers in the breathing zone. I’ve watched less experienced workers cough and rub their eyes when working outside ventilation, but those who use the tools at hand avoid the issue. Dedicated work benches with spill trays make cleanup simpler. Keeping incompatible materials separated cuts down on the chance of nasty surprises.
Simple barriers bring big peace of mind. Nitrile gloves outperform latex with many solvents and reagents, holding up when latex falls apart. Lab coats help shield personal clothing, and safety goggles are non-negotiable. If powder or vapor is possible, a dust mask or respirator helps guard against accidental inhalation. Closed-toed shoes keep splashes off your skin. Changing out gloves and washing hands before leaving the work area makes sure nothing travels home.
Spill kits packed with absorbent pads, neutralizing agents, and instructions save the day. Clear emergency routes paired with showers and eyewash stations make fast responses possible. I have never seen anyone plan to have an accident—but the ones who keep gear ready bounce back quickest. Posting contact numbers for poison control and supervisors keeps support close. Regular drills keep everyone sharp.
Treating hazardous waste by tossing it down the sink crosses a clear line. Labeled containers dedicated to organics or halogenated waste keep separation simple. Collaborating with approved hazardous waste disposal partners avoids fines and bigger environmental headaches. Inventory gets checked before purchase; buying smaller quantities helps avoid long-term storage concerns.
Building safer habits doesn’t happen overnight. It grows from sharing experience, staying informed, and keeping training ongoing. Every mishap teaches a lesson. Attention to detail and respect for process stop problems before they start. In chemistry, that sort of care isn’t petty—it’s professional.
Lab work has always demanded sharp attention, especially with chemicals like 5-Chloro-1-Methyl-1H-Imidazole. Even after years around chemicals, I never take shortcuts. This compound, used in pharmaceutical and research circles, can surprise anyone who gets careless. It deserves respect for what it can do – and what it can ruin if ignored.
Open a fresh bottle of this chemical and a pungent odor often hits the nose. Fumes can bother airways and eyes; long exposure makes irritation worse. These aren’t irritations to brush aside. Colleagues have dealt with coughing fits just from poor ventilation. Even skin contact, once brushed off as a minor risk, leaves rashes that linger for days. The risk scales with dose, but unpredictability usually wins out – some people react much worse than others.
Spills interrupt workflow and place everyone on edge. This liquid doesn’t mix kindly with bare hands or eyes. It also wants to evaporate. Heat in a cramped workspace means inhalation risk jumps. Cleaning a spill means using gloves and not just the thin disposable kind; splash-proof goggles and lab coats stand between you and a lost afternoon at the occupational clinic.
5-Chloro-1-Methyl-1H-Imidazole, while not as infamous as some solvents, still catches fire. Most labs keep clear labels about which chemicals pose ignition risks, but even so, stories circulate of colleagues who ignored the low flashpoint. If a spark lands nearby, ignition does not wait long. Water might not control small fires; dry chemical powder or carbon dioxide works better. Fire drills feel tedious until a flaming vial snaps everyone into focus.
Mixing this compound with strong oxidizers or acids can trigger reactions you won’t soon forget. The risk runs both ways: accidental mixing ruins experiments and also places staff at risk of burns or nasty fumes. Chemical storage matters; frustration shows up when someone puts incompatible materials in neighboring cabinets. Segregation and labeling systems take the guesswork out and help avert whole-room evacuations.
In my years at the bench, proper gear always makes the difference. Nitrile gloves last longer and actually block contact. Cheap latex sometimes lets traces slip through. Goggles with side shields beat regular glasses – splash injuries happen in a second and last for weeks. Lab coats, not street clothes, soak up the unexpected.
Good habits beat heroics. Wash hands after handling and before breaks. Open vials only in fume hoods. If fumes waft out, hit the exhaust and step back. Trust noses and eyes – symptoms do not make good warning signals since trouble often starts before they show up. I remember a vent failing during a late-night session; it irritated everyone’s throats, and we agreed never to skip those system checks again.
Never skimp on training, no matter how familiar the name. Refresher sessions, safety data sheet reviews, and even simple reminders about labeling keep mistakes rare. Secure storage, good house rules, and written cleanup steps all pay off. Spills or exposures show up less often when lab culture values readiness over speed. Being methodical works better than gambling with luck.
5-Chloro-1-Methyl-1H-Imidazole by itself does not demand fear, but it deserves focus and real preparation. Taking chemicals seriously simply means people get through the day without stories best left untold.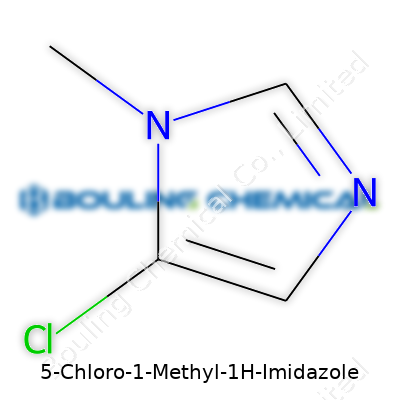
| Names | |
| Preferred IUPAC name | 5-chloro-1-methyl-1H-imidazole |
| Other names |
5-Chloro-1-methylimidazole 1-Methyl-5-chloroimidazole 1H-Imidazole, 5-chloro-1-methyl- 5-Chloro-N-methylimidazole 5-Chloro-1-methyl-1H-imidazole |
| Pronunciation | /ˈfaɪ-klɔːrə-wʌn-ˈmɛθ.ɪl-ɪˈmɪdəˌzoʊl/ |
| Identifiers | |
| CAS Number | 5165-78-8 |
| 3D model (JSmol) | `3D Structure (JSmol) string for 5-Chloro-1-Methyl-1H-Imidazole:` ``` CN1C=NC=C1Cl ``` |
| Beilstein Reference | 2811042 |
| ChEBI | CHEBI:134427 |
| ChEMBL | CHEMBL18936 |
| ChemSpider | 67410 |
| DrugBank | DB08319 |
| ECHA InfoCard | 03ee489d-8f6a-480e-9a8b-98c73135b34d |
| EC Number | 613-345-2 |
| Gmelin Reference | 505114 |
| KEGG | C19268 |
| MeSH | D016678 |
| PubChem CID | 69977 |
| RTECS number | NI1325000 |
| UNII | 2JXS7I4L3I |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | DVV21RQ4YO |
| Properties | |
| Chemical formula | C4H5ClN2 |
| Molar mass | 114.56 g/mol |
| Appearance | White to pale yellow solid |
| Odor | Odorless |
| Density | 1.24 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.11 |
| Vapor pressure | 0.5 mmHg (25°C) |
| Acidity (pKa) | 13.90 |
| Basicity (pKb) | 7.05 |
| Magnetic susceptibility (χ) | -38.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5320 |
| Viscosity | 0.88 cP (20 °C) |
| Dipole moment | 3.73 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -8.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3779.6 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P330, P337+P313, P362+P364, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 95 °C |
| Autoignition temperature | Autoignition temperature: 475 °C |
| Lethal dose or concentration | LD50 Oral Rat 670 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2000 mg/kg |
| NIOSH | RN8221 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 5-Chloro-1-Methyl-1H-Imidazole is not established. |
| Related compounds | |
| Related compounds |
1-Methyl-1H-imidazole 5-Bromo-1-methyl-1H-imidazole 5-Chloroimidazole 2-Chloro-1-methylimidazole 4-Chloro-1-methyl-1H-imidazole |