Chemists have spent decades learning to master brominated heterocyclic compounds, and (5-Bromothiophen-2-Yl)-Phenylmethanone traces its roots back to the evolution of organosulfur chemistry. Early work focused on foundational thiophene rings, recognized for their stability and unique electronics. The bromine atom’s addition gave new handles for reaction, making this molecule more than a lab curiosity. In the mid-to-late 20th century, laboratories began exploring benzoyl derivatives in organic synthesis. The brominated thiophene-benzoyl amalgam grew from this legacy, pushed forward by the increasing demand for fine specialty chemicals, especially in pharma and electronic materials. By the 1990s, the structure appeared more frequently in patent filings and journal articles, often explored for cross-coupling or as a scaffold in medicinal chemistry pipelines.
(5-Bromothiophen-2-Yl)-Phenylmethanone stands as a unique hybrid, blending the electronic effects of a phenyl group with the sulfur-rich thiophene and the selective reactivity of bromine. Yet, the molecule isn’t just laboratory eye-candy—labs depend on this compound for its ability to foster both small changes and sweeping transformations in target molecules. The synthesis of such compounds may seem simple on paper, but small shifts in process can have a big effect on purity and performance. Anyone who has handled these intermediates understands the critical interplay between reagent choice, solvent interactions, and workup conditions. Lab practice proves, time and again, that the details matter.
As a solid at room temperature, (5-Bromothiophen-2-Yl)-Phenylmethanone exhibits a crystalline appearance—sometimes off-white, sometimes bearing tinges from trace impurities that slip through even careful purification. With a molecular formula of C11H7BrOS and a molecular weight around 267.14 g/mol, its density shows the heft of a halogenated aromatic compound. The melting point typically falls near 62–66°C, though subtle shifts can reveal the presence of minor byproducts. Solubility in organic solvents like DMSO, DMF, chloroform, and dichloromethane makes it accessible for a range of synthetic operations. The molecule’s distinctive odor—rooted in its thiophene core—serves as a reminder that chemistry is always personal and sensory. Stability under normal storage makes it manageable, but the presence of the bromine atom requires some care around light, moisture, and heat.
Experienced chemists scrutinize technical sheets for specific features. Purity levels, determined by chromatography or NMR, often exceed 97%. Spectral data gives the confidence needed for downstream synthesis—peaks in the proton and carbon NMR match published values, while mass spectrometry confirms molecular integrity. Clear labeling includes CAS Number 50814-24-7, appropriate GHS hazard statements, batch number, production date, and recommendations for storage—typically cool, dark, and dry. Reliable suppliers add safety guidelines, MSDS access, and sometimes trackable lot histories to support transparency. These technical details anchor safe and reproducible research, creating a foundation for further modification.
Most synthetic routes start with a brominated thiophene substrate, which reacts with a benzoyl chloride derivative under Friedel–Crafts acylation conditions. Use of aluminum chloride, handled with care due to its moisture sensitivity, drives formation of the target aryl ketone. Variations exist—switching up protecting groups, temperature regimes, solvent systems—to optimize yield and purity. Anyone who has run these reactions knows that careful control of reaction time, temperature, and quenching sequence keeps byproducts at bay. Purification involves extraction, washing, and crystallization, or sometimes column chromatography, if moieties closely resemble each other. Yield management leans on both bench skills and a willingness to tinker with conditions until the spectrum reads clean.
The potential of (5-Bromothiophen-2-Yl)-Phenylmethanone in synthetic chemistry shows itself in each new transformation. The bromine atom opens the door for palladium-catalyzed coupling—Suzuki, Sonogashira, Heck, and Stille methods—where customized biaryls, alkynes, and other conjugated systems get assembled. Cross-coupling plays a starring role, but nucleophilic aromatic substitution, Grignard addition, or even reduction of the carbonyl provide more options to shape molecular architecture. The molecule’s stability under various conditions lets researchers experiment, fine-tuning reaction partners and solvents to match their ambitions. These pathways help both academic and industrial laboratories solve puzzles in pharmaceuticals, agrochemicals, and new-materials research.
In catalogs and literature, several names surface for this substance: 1-(5-Bromothiophen-2-yl)-2-phenylethanone, 5-Bromo-2-thienyl phenyl ketone, and 2-Benzoyl-5-bromothiophene all direct chemists to the same compound. CAS 50814-24-7 serves as a constant amid these synonyms, ensuring researchers order the right material. Understanding alternate nomenclature lowers the risk of mix-ups, especially when comparing results or sourcing raw materials.
Personal experience in the lab tells you a lot about safety—small amounts of dust in the air, unexpected reactivity, all demand respect. (5-Bromothiophen-2-Yl)-Phenylmethanone falls under GHS classification for skin and eye irritation. Wearing gloves, a lab coat, and eye protection stops problems before they start. Labs with a culture of safety always include efficient ventilation, regular MSDS reviews, and dedicated waste disposal methods for organohalogens and sulfur-containing debris. Regular audits remind staff to stay sharp. Emergency protocols, spill kits, and proper first aid supplies save time and prevent accidents when things go off script.
This compound stands out as a key intermediate in organic synthesis, bridging the worlds of pharmaceuticals, advanced polymers, and organic electronics. Medicinal chemists chase new drug candidates by leveraging this scaffold's ability to connect with bioactive fragments. Material scientists value the thiophene core’s role in conducting polymers, exploring new frontiers in OLEDs and organic solar cells. Agrochemical research taps into the reactivity too, using it as a launchpad for novel protection agents and growth regulators. The versatility seen in (5-Bromothiophen-2-Yl)-Phenylmethanone’s chemistry continues to attract teams pushing for better, safer, and more sustainable molecules.
Continuous improvement in chemistry depends on compounds like this one. Research teams often tackle yield optimization, greener process routes, and scaled synthesis—balancing cost against purity and performance. Collaboration between academic labs and industrial facilities speeds progress, with researchers sharing data on catalytic efficiencies, environmental impact, and end-use stability. Computational chemistry and AI support better predictive pathways, letting chemists focus experiments where success seems likely. Projects today often involve real-time analytics, feedback loops, and digital documentation, making it easier to learn from both successes and failures. Such initiatives bring the molecule’s potential to more arenas, fueling exploration in everything from improved antibiotics to high-performance batteries.
Laboratory vetting for safety always includes rigorous toxicity assessment. (5-Bromothiophen-2-Yl)-Phenylmethanone’s structure raises some red flags with its brominated and aromatic content, and toxicologists do not cut corners. Standard in vitro tests, using human and animal cell lines, map out cytotoxicity thresholds. Environmental impact, persistence, and breakdown products are tracked in parallel. So far, most reports suggest low acute toxicity, but the chronic effects—especially for off-target environmental organisms—demand more research. Personal lab work often requires close-out paperwork for any unexplored toxicity, and many research facilities prioritize disposal through licensed waste management partners, minimizing exposure risks to staff and surrounding ecosystems.
Looking at the road ahead, (5-Bromothiophen-2-Yl)-Phenylmethanone finds itself poised for a larger role as innovation demands new cross-coupling partners. The compound’s sturdy backbone and versatility keep it relevant as chemists develop greener, more efficient synthetic methods. Development of bio-based or recyclable process shortcuts could lighten the environmental footprint, meeting stricter regulations and market expectations for sustainability. Advances in medicinal and materials chemistry continue to highlight this compound, as researchers unlock fresh uses and derivatives. More robust toxicity studies, automated handling protocols, and digital synthesis platforms will likely shape the next chapters for this chemical, helping labs innovate safely, quickly, and with a clear record of stewardship.
Even in a fast-paced world brimming with technological shifts, organic chemistry keeps popping up in daily headlines. (5-Bromothiophen-2-Yl)-Phenylmethanone strikes as a good example. It’s got a name only chemists would love, yet its structure tells a story with roots in both medicine and technology. This compound draws interest for its versatility—showing up in research around pharmaceutical intermediates and materials chemistry.
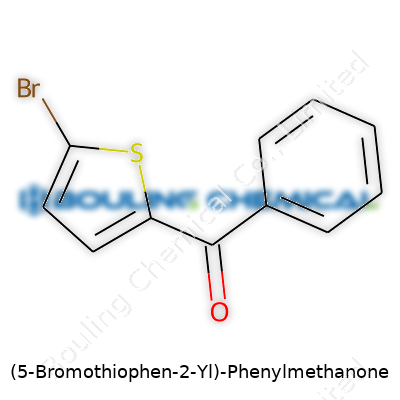
(5-Bromothiophen-2-Yl)-Phenylmethanone carries a backbone familiar to those who’ve spent even a little time in a chemistry classroom. At the heart: a benzene ring and a brominated thiophene ring, both of which connect through a carbonyl bridge. The molecular formula reads C11H7BrOS. It’s a mouthful, but it clarifies where each part fits.
Start with the phenyl group—a simple benzene ring bouncing with aromatic stability. Next, there’s a carbonyl group. That’s a carbon atom double-bonded to an oxygen, a point of attachment between the benzene and thiophene ring. On the other end, the thiophene ring comes with five members—four carbons, one sulfur. Here’s the twist: a bromine atom lands on the fifth position of the thiophene, giving this compound unique reactivity.
Scientists don’t just stare at structures for fun. The arrangement of atoms explains the properties, guiding hands in the lab and minds in industry. Adding a bromine to thiophene isn’t just a random move—it influences how this molecule behaves, how it absorbs light, reacts with others, or slots into larger synthesis pathways.
Consider the electron-rich nature of both the benzene and thiophene rings. Throw in bromine, an electron-withdrawing group, and you get a scaffold primed for further transformation. In practice, chemists use this kind of molecule to build more complex drugs, test new organic semiconductors, or craft dyes with precise optical features. I’ve seen more than a few students marvel at how a single atom, like bromine, can transform a humble molecule’s reactivity.
Making (5-Bromothiophen-2-Yl)-Phenylmethanone isn’t always as simple as mixing a couple of flasks. The key issue sits in controlling reactivity—bromine atoms can be fussy partners, making regioselectivity a big concern. Impurities often creep in, threatening to derail yield, purity, or even safety. Researchers often turn to palladium catalysts and careful temperature control to keep the synthesis on track.
Environmental safety matters, especially with brominated compounds. Strict protocols for handling and disposal help keep risks in check. In the lab, I’ve handled similar substances—there’s always an unmistakable sense of care, sleeves rolled down, fume hoods humming.
Chemists continue to search for cleaner, more energy-efficient ways to synthesize molecules like this. Green chemistry offers new solvents or milder reaction conditions; automation speeds up discovery, slashing trial-and-error. Open publication of procedures means even small labs can replicate big results. If the world wants safer medicine and smarter materials, understanding and tweaking molecules like (5-Bromothiophen-2-Yl)-Phenylmethanone is more than an academic exercise—it’s a cornerstone for real progress.
People in research labs usually keep a close eye on useful building blocks, and (5-Bromothiophen-2-Yl)-Phenylmethanone certainly counts as one of them. Medicinal chemists turn to this compound when they need a bridge between simple, small molecules and more complex ones — often for new drug candidates. The combination of a bromine atom with a thiophene ring and a benzophenone framework gives chemists plenty to work with. It can react in Suzuki, Heck, or Stille couplings, so it often finds a role as a starting point for pushing two different molecular fragments together. In drug discovery, the thiophene core crops up in antifungal, anti-inflammatory, and anticancer designs. Researchers keep testing different substitutions around this core, hoping to bump up the activity or reduce side effects for future medicines.
I’ve seen graduate students, postdocs, and engineers use (5-Bromothiophen-2-Yl)-Phenylmethanone to build blocks for organic semiconductors. The bromine group sticks out on the thiophene, making the whole molecule ready to snap onto other pieces. This makes it useful for pi-conjugated systems, which show up in organic light-emitting diodes (OLEDs), organic field-effect transistors (OFETs), and solar cells. You won’t usually see it show up in the final product, but the fragments built from this compound enable flexible electronics. People chasing higher conductivity or new optical properties often start with something like this, tinkering until they get the right structure for their next device.
Chemists trying to build new crop protection agents or herbicides test rings and chains of all kinds. The thiophene core in (5-Bromothiophen-2-Yl)-Phenylmethanone mimics features that show up in real-world pesticides. By manipulating substitutions at either the bromine or phenyl side, scientists can probe plant and pest biology, looking for new handles for control. Agricultural research never runs out of trial compounds, but finding a new backbone with good field performance doesn’t happen every year. This molecule brings options to the table—sometimes as a finished product, but more often as a hidden scaffold in bigger, more complex molecules.
Specialty chemical suppliers list (5-Bromothiophen-2-Yl)-Phenylmethanone in their catalogs because researchers need a ready supply for new ideas. Academic teams work out its transformations in methodology papers, figuring out better ways to link the thiophene ring to other molecular fragments. In industry, material scientists and med chem labs buy it to speed up process development, structure-activity relationship studies, or as a side-step when a particular reaction keeps failing. This versatility shifts the focus from just what the molecule does to how fast and reliably researchers can get new compounds into testing.
The potential for (5-Bromothiophen-2-Yl)-Phenylmethanone tracks with the speed of chemical innovation. When new reaction methods hit the literature—run under milder temperatures or with less toxic reagents—this compound often becomes part of the test set. Sourcing and scaling remain sticking points for larger batches, especially in drug or materials scale-up. Keeping production safe and efficient, with fewer hazardous byproducts, stays front of mind for chemists and manufacturers. Some research groups explore greener pathways for synthesis, focusing on lower-waste routes or recyclable catalysts. Cost and safety concerns keep companies on their toes, pushing for improvements all the time.
Anyone who has spent a few hours hunched over a lab bench or a spreadsheet knows the real challenge: numbers don’t lie, but they demand respect. Looking up the molecular weight of (5-Bromothiophen-2-Yl)-Phenylmethanone isn’t just a trivial task for chemists or students. It ties directly into why chemicals work the way they do, how reactions get scaled up, and how safety data sheets get written. Having spent years flipping through CRC handbooks and double-checking formulas (after a professor spotted one bad addition), I get why such numbers deserve more attention than most folks give them.
Each part of (5-Bromothiophen-2-Yl)-Phenylmethanone matters. There’s a phenylmethanone (benzoyl) group, a thiophene ring, and a bromine atom sitting at the 5-position. It’s a mouthful, but that extra precision decides everything from physical handling to regulatory reporting. Miss a single carbon, and the math leads down the wrong alley.
A molecular formula acts like a roster for a sports team: you tally up who’s on the field. For (5-Bromothiophen-2-Yl)-Phenylmethanone, you find C11H7BrOS. Gritty detail makes it clear:
Totaling it up lands on 267.14 g/mol. That’s not just a number for the shelf — it determines how many grams to weigh out in the lab, how a reaction is balanced on paper, and how shipping forms get filled out.
Mistakes in molecular weights cause headaches or, worse, real safety issues. My early years saw a batch ruined from a decimal point error. Reliable suppliers, trusted databases like PubChem or ChemSpider, and peer-checked journals build the confidence needed for decisions that keep experiments safe and budgets intact. A single trusted number pulls its weight across procurement, toxicology reports, and patent filings.
Researchers working on synthetic routes or pharmaceuticals know a minor change in a structure can mean weeks of lost work or regulatory hassle. The bromine atom affects more than the number on the scale; it tweaks toxicity, influences reactivity, and triggers more complex shipping rules. Environmental laws don’t cut slack for a rounded estimate or a lazy copy-paste. Every digit matters, and those digits rely on correct calculation.
Automation and software help, but trusting every screen drains accountability. There’s nothing fancy about double-checking a formula by hand before running with the molecular weight. Mixing human skepticism with digital output never goes out of style. Sound science keeps thriving on small details caught by careful people. Every correct calculation helps push safer, cleaner, and more reproducible chemistry, no matter the setting.
Working in labs, I’ve seen chemicals handled with care and with neglect. A compound like (5-Bromothiophen-2-Yl)-Phenylmethanone isn’t something to treat lightly. Every container on a shelf carries a story of what could go right and what could go wrong. This substance sits among aromatic ketones, used in research—and, as with many organic synthons, people often overlook the details once they stow it away. Keeping it safe goes far beyond locking it in a cupboard.
Most failures in chemical storage start with temperature. (5-Bromothiophen-2-Yl)-Phenylmethanone prefers a cool, stable environment. Temperatures above 25°C let reactive impurities break down or, worse, invite volatility. My own experience tells me: don’t trust ambient lab temperature, especially in summer months or in places with unreliable air conditioning. Chemical refrigerators, set between 2 and 8°C, offer a tighter grip on safety. Direct sunlight does more harm than good, accelerating decomposition and creating unknown byproducts. I always find a dark place, away from window glare and fluorescent bulbs.
Humidity looks harmless, but it creeps into containers and slowly wrecks sensitive compounds. This ketone doesn’t like damp air. I watched a colleague lose a batch because a desiccator was left open overnight. Silica gel or other drying agents inside a desiccator container keep ambient moisture at bay. Make sure lids seal tight—one loose cap, and you’re out an entire purchase order.
Lab safety officers remind us: never store unlabelled or unsegregated bottles. (5-Bromothiophen-2-Yl)-Phenylmethanone doesn’t play well with oxidizers or strong bases. I keep it on a shelf with compatible aromatics, far from bleach or caustic soda. Segregating chemicals by hazard class earns more than a checkbox on a safety audit. It protects everyone coming through the door. Personally, I’d rather triple check a shelf plan than risk an incident.
The material of the storage container shapes outcomes. Amber glass bottles block out light and don’t react with the compound. I stay away from cheap plastics—solvents and organics can leach or cause cracking. A solid screw cap with no visible wear outperforms a snap-top every time. Attach a durable label: full chemical name, date, and any hazard warnings. It takes no more than a minute, but pays off in emergencies and audits.
No one expects a spill, yet labs see their fair share. Absorbent pads and PPE—gloves, goggles, lab coats—make the difference between a minor hiccup and a serious hazard. I once witnessed a small leak caught early thanks to proper containment trays under the shelf. Secondary containment is more than redundancy; it’s protection against inattentive moments. Training every staff member on these protocols stops a small issue from escalating.
Safe storage isn’t theory; it's daily work. Review inventory monthly. Check expiry dates and crystal growth. Refresh drying agents. Report missing labels or poor seals at once, not tomorrow. Institutional culture can make or break lab safety, and lives often depend on these everyday routines.
Walking into any lab means encountering a collection of bottles with difficult names, labels covered with warnings, and a silent understanding that knowledge protects more than latex gloves ever could. (5-Bromothiophen-2-yl)-phenylmethanone fits right into that line-up. This compound, a brominated ketone with both an aromatic ring and a sulfur bearing group, pops up in organic syntheses and research across specialty chemistry. Its structure combines a bromine atom with a thiophene and a benzoyl group, making it useful as a building block. Still, handling it brings questions worth exploring.
Any chemical with halogenation raises a flag. Bromine atoms make organic compounds heavier and often more reactive. In my own experience, brominated lab compounds sometimes give off a biting, acrid odor, and more than once I've felt a slight burn after a few careless touches – despite gloves. Even if (5-bromothiophen-2-yl)-phenylmethanone isn’t as aggressive as elemental bromine, it’s smart to expect skin and eye irritation if contact happens. Material safety data sheets for similar compounds recommend working in a fume hood, keeping it off skin, and never, ever breathing in dust or vapors. Most labs ban eating and drinking near such substances for good reason.
Beyond personal irritation risk, there’s the environmental angle. Brominated aromatics sometimes hang around longer than simpler molecules. Their breakdown products might not be friendly. Anything tracking out of flasks or spilling on benches must stay contained, and waste calls for special disposal, not a quick rinse down the drain. This holds up, chemically and ethically. A little bit can go a long way, in both synthesis and accidental spread.
Direct studies on (5-bromothiophen-2-yl)-phenylmethanone itself remain limited, typical of many specialty intermediates. Still, looking at what happens with similar compounds gives a clear enough picture. Many aromatic ketones cause irritation, and brominated organics tend towards toxicity at higher doses. Some even build up over repeated exposure. NIOSH and OSHA agencies don’t specify rules for every compound, but relying on broad aromatic and halogenated compound handling standards works best. That means goggles, nitrile or butyl gloves, proper ventilation, and careful accounting of every gram brought into the work space.
Anyone working with this compound gets sharp on habits quickly. Open the bottle only in a working fume hood. Label everything – and skip the hand-written sticky note. Double glove for transfers where splashing or spills could happen. Leave a spill kit within arm’s reach, along with material safety sheets. Make sure one’s lab mate knows if an experiment turns sour, not just for their sake, but because medical teams need to know what’s involved if something goes wrong.
For those less familiar with chemical hazards, training and mentorship make a world of difference. Reading up on chemical safety only goes so far without someone pointing out what “best practice” looks like in action. Checklists posted near benches might seem basic but can save a lot of trouble on busy days. Waste solvent containers require special labeling, and the container stays capped until filled, never left open.
The world doesn’t stop using specialty chemicals just because they bring risks. The real trick lies in respect for what sits inside the bottle. Safety grows from routine, vigilance, and taking small moments to double check before getting to work. Labs might never reach zero risk, but with every lesson, poster, and glove change, that margin of safety gets just a bit wider.
| Names | |
| Other names |
(5-Bromo-2-thienyl) phenyl ketone 2-Benzoyl-5-bromothiophene 5-Bromo-2-benzoylthiophene |
| Pronunciation | /ˌfaɪvˈbroʊmoʊˌθaɪəˌfinˈtuːɪlˌfɛnɪlˈmɛθəˌnoʊn/ |
| Identifiers | |
| CAS Number | 65694-21-1 |
| 3D model (JSmol) | `3D JSmol string: CC1=CC=C(C=C1)C(=O)C2=CC=CC=C2Br` |
| Beilstein Reference | 120595-35-1 |
| ChEBI | CHEBI:67523 |
| ChEMBL | CHEMBL3704688 |
| ChemSpider | 123785 |
| DrugBank | DB07670 |
| ECHA InfoCard | 100.105.628 |
| EC Number | EC 693-344-5 |
| Gmelin Reference | Gmelin 86727 |
| KEGG | C21775 |
| MeSH | D000000 |
| PubChem CID | 68440509 |
| RTECS number | GX7920000 |
| UNII | 4O7K6176E8 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID1067598 |
| Properties | |
| Chemical formula | C11H7BrOS |
| Molar mass | 291.17 g/mol |
| Appearance | Off-white solid |
| Odor | No characteristic odor |
| Density | 1.551 g/cm3 |
| Solubility in water | Insoluble |
| log P | 2.9 |
| Acidity (pKa) | pKa = 7.6 |
| Basicity (pKb) | 12.65 |
| Magnetic susceptibility (χ) | -73.93 × 10^-6 cm³/mol |
| Refractive index (nD) | 1.689 |
| Dipole moment | 2.99 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 369.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1741 kJ/mol |
| Hazards | |
| Main hazards | H302 + H315 + H319 + H335 |
| GHS labelling | GHS02, GHS07 |
| Pictograms | `Nc1ccsc1Br` |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P270, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-2-1 |
| Flash point | > 150°C |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 10 μM |