Chemists got curious about benzothiophene derivatives well before powerful NMR spectrometers filled labs. Back in the early 20th century, researchers started exploring fused aromatic systems aiming to uncover new reactivity and building blocks for the dye and pharmaceutical space. 5-Bromobenzothiophene emerged as a targeted variant in that hunt, as halogenated aromatics branched into different application spaces. Traditional halogenation of benzothiophene, especially at the 5-position, took off in the mid-1900s—with improvements in selectivity following right after. These developments helped move from laborious batch syntheses to more scalable and reliable approaches, making this compound accessible on larger scales necessary for both academic and industry research.
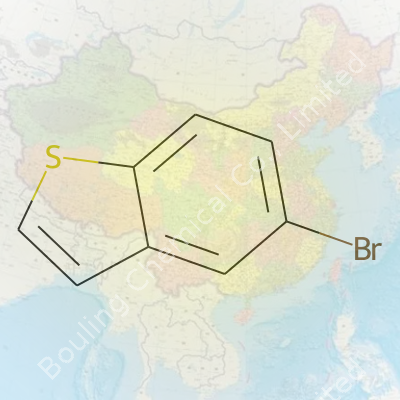
5-Bromobenzothiophene draws attention as a key intermediate in organic synthesis. Its structure—a benzothiophene ring bearing a bromine at the 5-position—gives it unique reactivity and compatibility with several cross-coupling reactions. Labs value it as a solid platform for modifications, mainly for the introduction of further substituents or for building more complex molecules. Suppliers ship it as a crystalline solid, usually in sealed glass or plastic containers suitable for moderately air-sensitive chemicals. On the bench, it stands out for its adaptability in both research and small-scale industrial settings.
5-Bromobenzothiophene brings a pale-colored crystalline appearance, sometimes tending slightly off-white depending on purity and storage. Its molecular formula is C8H5BrS with a molecular weight around 213.1 g/mol. Melting point hovers between 51 and 54°C. The compound resists water and most polar solvents, dissolving best in organic options like dichloromethane, toluene, or THF. The presence of bromine not only adds weight but also alters its reactivity, especially toward metal catalysts and in nucleophilic aromatic substitution. These features allow chemists to control reactivity in a predictable, stepwise fashion.
Bottles from commercial suppliers tend to specify purity—often in the range of 97% or above—with impurities listed by HPLC or GC-MS analysis. Labels include the CAS number 1003-09-4, hazard pictograms (mainly for irritants), and handling notes. Storage advice generally stresses keeping the bottle tightly sealed, dry, and out of intense light. Handling guidelines appear directly on the packaging, warning of dust inhalation and skin contact risks. Lot numbers and batch analytical certificates back up quality claims, providing researchers with traceability.
People usually start synthesis with benzothiophene and subject it to direct bromination, typically using N-bromosuccinimide (NBS) in a solvent like acetonitrile or DMF. Reaction temperatures hover just above room temperature, promoting selectivity for the 5-position over other reactive sites. After stirring for several hours, workup involves quenching with aqueous sodium thiosulfate, extraction into a nonpolar solvent, and crystallization or chromatography for purification. Optimizations over the years have cut byproducts and boosted yield, reducing waste conversation—a significant improvement over older batch processes involving elemental bromine and harsh conditions.
Chemists rely on the bromo group’s chemistry for further functionalization. Suzuki-Miyaura or Stille couplings replace the bromine with aryl, vinyl, or alkynyl groups, crafted to yield specialty materials or pharmaceutical precursors. Lithiation strategies give direct access to lithiated intermediates at the 5-position, followed by quenching with electrophiles for versatile modifications. Electrophilic aromatic substitution, on the other hand, allows further halogenations or nitrations, often tuned for more elaborate scaffolds. The ability to pivot from 5-bromobenzothiophene into a broad range of derivatives puts it in high demand wherever controlled selectivity and modularity matter.
In catalogs and literature, 5-Bromobenzothiophene goes by several aliases. The IUPAC name is 5-bromo-1-benzothiophene, sometimes shortened to 5-bromo-2,3-benzothiophene. Common trade listings might simply use Benzothiophene, 5-bromo-, or reference its CAS: 1003-09-4. Researchers occasionally use abbreviations or shorthand, especially in synthetic schemes: 5-BBT is not unusual in notebook shorthand.
As with most halogenated aromatics, skin and eye irritation poses a risk, so nitrile gloves and eye protection remain on deck. Ventilated hoods take priority during weighing and transfers since inhaling dust leads to respiratory discomfort or worse. Emergency response sheets cite standard hydrocarbon solvent risks, so labs prioritize proper labeling and spill kits. Waste disposal follows guidelines for halogenated organics—no pouring down the drain. The compound’s thermal stability holds up for normal synthetic manipulations but breaks down upon sustained heating or strong acid/base exposure, putting routine thermal cycling off the table. Operational SOPs keep the focus on safe, contained workflows—especially critical with scale-up reactions.
Pharmaceutical researchers chase after benzothiophene derivatives for anti-tumor, anti-inflammatory, and CNS-active drugs, and 5-bromobenzothiophene delivers as both an end and a means to new structures. Material scientists have used it in the preparation of conjugated polymers, aiming for improved charge mobility and specialized optical properties in organic electronics. Agrochemical pathways also dip into its chemistry for crop protection candidates. In my own time on synthetic benches, this compound has shown up often for building block work in medicinal chemistry, where every new substituent can change a molecule’s fate. Its role in developing new dyes, sensors, and photonic materials has also gained traction, aligning with the trend toward smart materials.
Looking over recent R&D efforts, people are figuring out greener, more sustainable bromination protocols, focusing on minimizing hazardous byproducts and solvent waste. Innovations in continuous flow chemistry stand to reshape how labs access these aromatic platforms—reducing reaction times, improving safety, and tightening control over product distribution. High-throughput platforms screen new derivatives quickly, speeding up the feedback loop between synthesis and biological activity. In the literature, I’ve seen new catalyst systems push coupling efficiency, shrinking reaction temperatures and broadening functional group tolerance, a game-changer in medicinal chemistry and polymer science.
Animal studies and cell assays tell part of the toxicity story. Data suggest moderate acute toxicity in rodents, mainly at high oral dosages, with liver enzyme modulation as a typical biomarker. Skin and eye contact in lab animals tracks closely with what chemists experience—dose-dependent irritation but rarely severe systemic effects at typical exposure levels. Modern risk assessments push for alternatives to traditional animal-based evaluation, looking instead at in vitro predictions and computational modeling to map out hazard profiles. Regulatory databases catalog 5-bromobenzothiophene as a compound to use with caution, especially since comprehensive chronic exposure data don’t stretch as far as researchers would like. Labs keep exposure low, focusing on personal protection and rapid cleanup of spills to cut potential hazards.
As the demand for highly functionalized aromatics stays strong in drug and materials development, 5-bromobenzothiophene stands as a launchpad for discovery. Advances in catalysis unlock more creative pathways, reducing reliance on heavy-metal reagents and outdated halogen sources. Emerging fields such as organic electronics and next-generation photovoltaics call for even more diverse benzothiophene derivatives, and this compound serves as a reliable starting point. As computational chemistry marries with synthetic methods, prediction-guided design could help decide the next blockbuster structure. Regulations may tighten, demanding stronger safety evaluation and greener preparation protocols, but these challenges often spur innovation rather than slow it. In both academia and industry, curiosity around 5-bromobenzothiophene’s untapped chemistry keeps growing, and that’s how new breakthroughs start taking shape.
Ask anyone trying to build a molecule or run a reaction: purity is more than just a number on a bottle. With 5-Bromobenzothiophene, purity speaks to how easily a chemist can trust the results coming out of the flask. A chemist sitting in a lab, weighing out a portion of 5-Bromobenzothiophene, isn’t just tossing white powder into a solution—they’re betting on predictability. If this compound comes with hidden impurities, wasted time and money quickly follow.
Most lab suppliers offer 5-Bromobenzothiophene in purities ranging from about 95% to over 99%. Those extra few percent can make a surprising difference—especially in pharmaceutical research, or materials science, where trace contaminants can throw off results or add noise to data. Impurities don’t just change numbers; sometimes they change entire stories. A reaction that works with “high purity” can sputter out or give odd side products if the stuff from a cheaper source comes with 94% purity and a batch of unknowns.
Years back, I watched a colleague chase a mysterious side reaction for weeks before discovering the culprit was a sneaky impurity, not his own technique. He bought a cheaper sample labeled “95%,” thinking the margin wouldn’t matter for a routine screen. He ended up debugging it like a broken computer, only to order a 99% pure sample, and suddenly the problem vanished. That's a familiar story in research—shortcut on purity, pay on the other end.
Suppliers rely on techniques like HPLC (High Performance Liquid Chromatography) and NMR (Nuclear Magnetic Resonance) to check and report purity. Those machines don’t just look at the main ingredient—they pick up the odd solvents, side-products, or bits from the manufacturing process. Most catalogues list this data as “98%” or “99%” pure by HPLC—seems simple, but those few percentage points are a red flag or green light, depending on the next steps.
Making 5-Bromobenzothiophene isn’t a one-step affair. Each stage—from bromination to workup—can leave traces. Maybe the starting thiophene carried over, or a bit of brominated byproduct slipped through. Handling and storage play their part. Leaving the powder in a slightly damp warehouse or letting air seep in will introduce stuff you didn’t want.
In real-world research, those impurities don’t stay invisible. If the target is drug discovery, the wrong impurity can spark off odd biological results or toxicity signals in screens. In organic electronics, those trace chemicals can shift color or knock down performance. I’ve seen projects stall for weeks over small contaminants that crept in, only noticed after hours of frustrating troubleshooting.
Accurate paperwork matters for every bottle. Always check the Certificate of Analysis from a supplier before signing off. For critical work, some labs purify commercial samples themselves, using chromatography to bump that figure even higher. It’s about controlling variables—every unknown impurity adds another layer of confusion.
If budgets are tight and only basic testing is planned, maybe a 95% sample will do just fine. If the readout matters, that 99% sticker buys peace of mind. Plenty of labs find their balance with in-house quality checks or by sticking with brands that don’t cut corners. Either way, purity isn’t a number to ignore, and for 5-Bromobenzothiophene, smarter sourcing saves headaches down the line.
I’ve spent hours poring over chemical labels in labs that could double as meat lockers or ovens, so I know firsthand—storage really can make or break a chemical’s shelf life. 5-Bromobenzothiophene’s name may stretch across a page, but its storage rules read more like common sense than high science, provided you respect what’s actually in the bottle.
Moisture causes more headaches in the chemistry world than just about any other factor. 5-Bromobenzothiophene doesn’t handle water well, so a tight-sealing container is non-negotiable. Labs that take shortcuts with leaky jars or crumbling lids soon discover sticky messes or strange colors, both of which shout, “Ruined sample!” Once water’s in, you’re cleaning up, not running experiments.
Dessicants—those little packs people toss by mistake—belong in the same box as the sample itself. They draw out stray moisture and earn their keep in humid climates. In tropical labs I’ve seen, storing sensitive reagents without such moisture control turns work weeks into waste. So it’s always glass, always airtight, and most times, a fresh dessicant inside the jar.
I once left a bottle under a fluorescent bench lamp for the afternoon and came back to find the label’s ink faded—and the powder inside slightly offshade. Direct light, especially UV, loves to break bonds in aromatic compounds. 5-Bromobenzothiophene itself can react if given the chance. Cabinet doors or amber-tinted bottles are the standard fix—a simple shelf, far from windows, works just as well. Chalk this up to years of seeing yellowed bottles in sunlight and writing off batch after batch.
Many organic compounds get cranky with heat. Whether you’re talking about sulking decomposition or plain volatility, anything above room temperature’s asking for trouble. For most of us, that means storing 5-Bromobenzothiophene in a cabinet away from radiators, ovens, and heaters—possibly climate-controlled if you’ve got the gear. In my cramped university lab, the “cool and dry” mantra meant keeping bottles at desk height, never on upper shelves where warm air collects.
Separation counts almost as much as temperature or dryness. Some chemicals put off fumes or vapors, others corrode caps. Chucking everything together in a hurry sounds convenient, but it invites disaster—not all reactions need a human hand to get started. In a storage cabinet, I find one shelf for sulfur-containing compounds like 5-Bromobenzothiophene, away from acids and bases. I’ve watched careless storage cut into budgets and put folks at risk, whether from air exposure or surprise reactions.
Give every bottle a clear, accurate label: date received, source, expiration if listed, and anything unusual you notice during handling. More bottles go to waste because of confusion than actual spoilage. In shared spaces, I tape up reminders and color codes. That extra minute saves hours later hunting through logs or, worse, responding to mysterious smells from an unknown sample.
An organic chemist’s best habits never really change. I store 5-Bromobenzothiophene in a cool, dry, dark place—airtight glass, marked up, with a dessicant pack inside. I do weekly checks and keep incompatible chemicals apart. If there’s ever any doubt how fresh a sample is, it’s replaced before risking a ruined experiment.
Chemical storage is less about memorizing rules, more about respect for a bottle’s quirks and an honest look at your own workspace. Skip the shortcuts, and the shelf life looks after itself.
Anyone who has wandered through a chemical stockroom will recognize the stacks of oddly spelled bottles, most of them far from household names. 5-Bromobenzothiophene sits among this crowd, looking unremarkable. Scratch the surface, though, and you’ll find that chemists prize this building block for what it can give them: new ideas for tackling real-world scientific problems.
When pharmaceutical companies hunt for new drug candidates, a reliable toolbox helps them piece together potent molecules. 5-Bromobenzothiophene stands out in this process. This molecule’s unique structure lets chemists swap, add, or tweak different pieces in the hunt for drugs targeting cancer, infection, or nervous system disorders.
Research teams often use it to create molecules that interact with proteins in the body, especially those in cell signaling or growth pathways. Drugs developed from benzothiophene scaffolds have eventually treated breast cancer and seizures. Bromine atoms built into the structure add flexibility, making it easier to attach more complex chemical forms, which can lead directly to patent-worthy discoveries.
In the world of electronics, steady progress depends on molecules that behave in predictable ways. Organic LEDs, semiconductors, and even new types of solar cells lean on molecules that can move electricity without breaking down. Chemists looking for alternatives to silicon have tried many routes, and 5-Bromobenzothiophene provides a promising starting point.
The sulfur in the benzothiophene ring brings certain electrical properties that scientists exploit to design new conductors and sensors. By adding a bromine atom, researchers gain a handle for further modifications — sort of like an anchor point for building fancier, more tailored molecules. Some of these creations help in flexible screens, or as sensing films that change when exposed to chemicals in the air.
Experimenters who love puzzles get pulled in by molecules like this. 5-Bromobenzothiophene gives researchers a shortcut — a way to add complexity, or to try out reactions that would otherwise take longer and cost more. The compound participates in cross-coupling reactions, especially Suzuki and Stille methods, which help join carbon atoms together cleanly.
This opens doors in creating complicated aromatic rings, structures at the heart of many substances that touch everyday life, from dyes and perfumes to new polymers. Students and experts alike rely on this compound as a dependable ingredient for exploring unknown chemical territory.
Real progress often slows because rare chemicals cost a lot or come with supply headaches. 5-Bromobenzothiophene hasn’t dodged these realities. Availability, purity, and environmental pressure all play a role in shaping how companies and labs use it. Looking to the future, sustainable production and greener methods for handling and transforming this compound will matter more than ever.
Chemists blend practical concerns with curiosity. They look for new reactions that might shave costs or use fewer harsh chemicals. Some turn to catalysis with less toxic metals. Others aim to recycle spent molecules wherever possible, reducing the drug discovery burden on the environment.
By looking at how compounds like 5-Bromobenzothiophene get used across industries, it becomes clear that chemistry, creativity, and constraint all intertwine. Each time a new application is found, it’s another puzzle piece for tackling problems in medicine, materials, and technology.
Walking into any chemistry lab, I’m always reminded how common it is to see molecules that don’t make the news but quietly carry out essential work. Take 5-Bromobenzothiophene. This name might sound like a tongue twister, but for research chemists, it’s simple business. Its molecular formula, C8H5BrS, explains the basics: eight carbon atoms, five hydrogen atoms, a bromine, and a sulfur. The molecular weight clocks in at about 229.10 grams per mole, a figure that usually guides practical lab work, especially for those mixing precise amounts for reactions or material synthesis.
Every time I’ve worked with halogenated aromatics in the lab, safety data and purity checks become a daily routine. The presence of both bromine and sulfur in its structure opens doors for creative syntheses. Researchers use molecules like 5-Bromobenzothiophene to build more complex compounds. I’ve seen it act as a stepping stone for pharmaceuticals or organic electronics, where both the electronic effects of bromine and the versatility of the thiophene ring play key roles.
Someone outside of lab work or chemical manufacturing might glance past this chemical, but the story doesn't end on a lab bench. In the world of organic semiconductors, chemists search for building blocks that mix stability with flexibility in reactions. The benzothiophene ring structure regularly shows up in organic LEDs and solar cells. By swapping out hydrogens for a bromine atom, you tweak its behavior in subtle but important ways. It’s a good reminder how small changes on the atomic level ripple up to large changes in performance. During my postgraduate work, the difference a single atom made in a reaction’s outcome sometimes caught the whole research group off guard.
Working with chemicals containing bromine comes with baggage. Sometimes it’s environmental restrictions, sometimes it’s sourcing costs. More than once, I’ve seen researchers wince at a chemical’s price tag following changes in regulations or supply chain hiccups. There’s also a push in the research community to find alternatives when possible, especially for compounds that bring environmental hazards with them. Everyone wants new materials, but few want long-term waste problems. Awareness of green chemistry practices becomes important. Some labs seek ways to recycle or safely decompose brominated byproducts, and supporting research into benign replacements for bromine is gaining ground.
While 5-Bromobenzothiophene rarely makes headlines, it tells a bigger story about the way a single molecule connects bench research, global supply chains, and environmental responsibility. Getting the molecular formula and weight right is just step one. Downstream, choices made at a molecular level end up influencing products, waste streams, costs, and technical progress in unexpected ways. Every research project I’ve been on involving these types of chemicals underscored the importance of both accuracy and thoughtfulness. Building a safer, more efficient future often runs straight through the center of compounds like this, where every atom has its part to play.
There’s no shortage of problems in chemical manufacturing, but the solutions start with transparency. Companies tracking not just their outputs but their feedstocks and waste streams seem better prepared for regulatory changes. Sharing successful recycling techniques or safer alternatives with the wider community boosts everyone. Education helps too—students who understand both the practical and environmental impact of every substance are the ones who eventually set better policies. And across research labs, having solid information about molecular weights and formulas allows people to focus energy on designing smarter, more sustainable reactions, instead of fixing basic mistakes.
The chemical world depends on proof. Whether you manage a busy lab, teach students, or run a startup in materials science, you’ve felt that moment of doubt when a new bottle arrives. Every reagent tells a story—only with a Certificate of Analysis (COA) does it tell that story clearly. If you purchase 5-Bromobenzothiophene, a COA isn’t just a formality. It’s a trust bridge spanning from manufacturer to user, laying out purity, moisture content, appearance, structure, and other critical markers.
As someone who has spilled half her coffee over inventory logs, I know the limited patience researchers have for paperwork. But in the middle of a synthesis, you depend on what’s in those logs. I remember using a reagent “off the record”—no COA, sketchy label, big regret. Weeks later, every result looked off, and tracking those errors led me straight to that one bottle. In labs, accuracy is only as strong as your weakest reagent.
For 5-Bromobenzothiophene, the importance of a COA jumps out when you realize where it travels next. Medicinal chemists tinker with it for drug precursors. Material scientists push it into new polymers and compounds. If the batch carries even a tiny trace of the wrong impurity, entire months of work drift out the window. The cost isn’t just money—it’s time, trust, reputation, and results.
A decent COA should dig deeper than just “99.0% pure.” It lists spectroscopic data, lots and batch details, and the date of analysis. Sometimes it catches things you didn’t think to ask about. Last year, I ordered a shipment from a supplier I’d never used. The COA flagged an unusual melt point. That raised enough doubt for me to rerun my own checks—and sure enough, the supplier had switched manufacturing processes, causing the product to degrade if stored for too long. That single page saved my deadline and a chunk of my sanity.
Not every supplier tosses in a COA by default. Some only provide one if you remember to request it—and sometimes they charge extra. That’s a problem. There’s no reason transparency around chemical quality should hide behind extra fees or awkward customer service. If you’re in the market for 5-Bromobenzothiophene, the first question should always be: “Can you show me the COA?” Shoppers prize free samples and fast shipping, but missing documentation leaves you exposed.
I’d like to see industry-wide norms shift. Suppliers who offer digital documentation portals or one-click downloads gain a following fast. Compliance officers and lab managers trust open records. It also smooths over audits and keeps insurance minimal. And if you’re a smaller startup, you don’t want your credibility shredded by something as basic as missing paperwork.
If you buy, make the COA non-negotiable. If you sell, bundle it automatically—no upcharge, no hassle. Many headaches vanish if everyone agrees the COA rides with the product every single time. This isn’t a call for more rules, just clearer expectations. Good science starts with knowing exactly what’s in your bottle.
| Names | |
| Preferred IUPAC name | 5-bromo-1-benzothiophene |
| Other names |
5-Bromo-1-benzothiophene 5-Bromo-benzo[b]thiophene |
| Pronunciation | /faɪˌbroʊmoʊˌbɛnzoʊˈθaɪoʊfiːn/ |
| Identifiers | |
| CAS Number | 1003-09-4 |
| Beilstein Reference | 1207525 |
| ChEBI | CHEBI:88844 |
| ChEMBL | CHEMBL19003 |
| ChemSpider | 141715 |
| DrugBank | DB08307 |
| ECHA InfoCard | 05e49aac-c777-4692-bd2a-47b816122387 |
| EC Number | 821-286-7 |
| Gmelin Reference | 1267559 |
| KEGG | C11083 |
| MeSH | D017912 |
| PubChem CID | 70127 |
| RTECS number | CG5425000 |
| UNII | Q7D3R61E5W |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C8H5BrS |
| Molar mass | 271.13 g/mol |
| Appearance | Off-white to light brown solid |
| Odor | Aromatic |
| Density | 1.60 g/cm³ |
| Solubility in water | Insoluble |
| log P | 3.9 |
| Vapor pressure | 0.00313 mmHg at 25°C |
| Acidity (pKa) | pKa = 0.71 |
| Basicity (pKb) | Basicity (pKb): 5.20 |
| Magnetic susceptibility (χ) | -82.3·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.683 |
| Dipole moment | 2.99 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 367.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4843.3 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P264, P271, P301+P312, P305+P351+P338, P405, P501 |
| Flash point | 144°C |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2000 mg/kg |
| NIOSH | QN8225000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 20 mg |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Benzothiophene 6-Bromobenzothiophene 5-Chlorobenzothiophene 5-Iodobenzothiophene 5-Nitrobenzothiophene 5-Methylbenzothiophene |