Scientists first started focusing on benzimidazole derivatives in the early 1900s, drawn by their unique chemical backbone and growing evidence of biological activity. In the 1930s, interest intensified after researchers linked compounds in this class to vitamin B12 biosynthesis, ultimately highlighting 5,6-dimethylbenzimidazole as a crucial part of the vitamin's structure. Since then, research teams worldwide probed its functions and production, with each decade bringing improvements in synthesis and an expanding portfolio of uses in nutrition, medicine, and analytical chemistry. Today, decades of accumulated knowledge provide a solid base for pushing its capabilities even further.
5,6-Dimethylbenzimidazole presents as a fine, pale crystalline powder. Laboratories rely on its high purity for both small-scale biochemical studies and large-scale industrial processes. Companies package it in sturdy, air-tight containers to limit moisture pick-up and preserve its shelf life. Scientists and engineers prize this compound not only for its essential role in vitamin B12 formulations but also for its versatility as a building block in synthesizing advanced agrochemicals, pharmaceutical intermediates, and even special dyes.
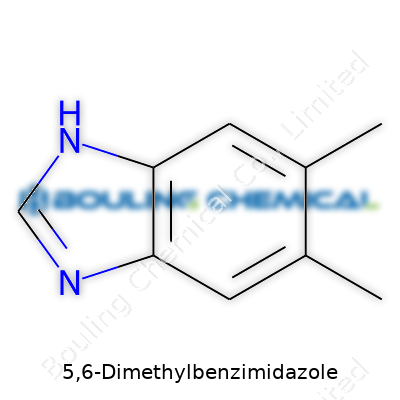
This compound features a melting point hovering around 195–198°C, confirming its resilience to mild heating. It dissolves sparingly in cold water, showing better solubility in organic solvents such as methanol and ethanol, which matters during extraction and purification steps. The molecule's structure, with methyl groups at the 5 and 6 positions of the benzimidazole ring, adds subtle electronic effects, creating interesting reactivity not seen in unsubstituted benzimidazoles. Chemists pay close attention to these details, as they influence how the compound reacts in further syntheses and how it behaves within biological systems.
Producers standardize technical specifications tightly. Each batch typically reports assays near 99% purity, with detailed certificates covering moisture, residual solvents, and trace impurities. For critical applications in diagnostics or clinical research, manufacturers back up their claims with chromatographic purity profiles, documented through HPLC or GC-MS. Labeling lists not just the chemical name but also alternative identifiers and hazard warnings, such as GHS pictograms if relevant, so users can handle it with confidence at every step.
The main route begins with o-phenylenediamine, which reacts with acetic acid and methylating agents to build the benzimidazole ring and add methyl groups at the right positions. To boost yields and minimize byproducts, careful control of temperature and reagent proportions becomes crucial. Some labs now favor greener solvent systems and catalytic methylation options, striving to reduce waste streams and limit operator exposure to hazardous reagents. These innovations often reflect direct conversations between chemists and plant operators—real-world teamwork that keeps industrial processes both safe and sustainable.
5,6-Dimethylbenzimidazole reacts cleanly with electrophilic agents, which allows chemists to tailor side chains and attach functional groups for specific projects. It handles bromination, nitration, and alkylation with predictable selectivity, paving the way to more complex heterocycles or use as anchors in bioconjugation. In drug development, medicinal chemists often exploit its nitrogen atoms to form strong hydrogen bonds—a subtle feature that can lock a compound into place when targeting enzymes or receptors.
Users might encounter a range of synonyms in literature and supply catalogs: 5,6-dimethyl-1H-benzimidazole, DMBI, and its IUPAC name 5,6-dimethyl-1,3-benzimidazole. Trade names do crop up in commercial vitamin blends or specialized reagents. Being fluent in these names makes it easier to source consistent material and interpret academic or industrial reports without confusion, which can prevent costly mix-ups in fast-paced work environments.
Like much of small-molecule organic chemistry, responsible handling depends on robust protocols and clear communication. Technicians never skip gloves and eye protection, especially during synthesis and transfer. Dust management keeps airborne exposure to a minimum; effective fume hoods and personal masks limit inhalation during weighing or blending. Companies who train their staff in safe material handling, spill control, and emergency cleanup consistently see fewer incidents. Regular reviews of SDS documentation and process risk assessments form daily routines in labs and production settings.
Nutrition and health remain key sectors: 5,6-Dimethylbenzimidazole’s major claim to fame comes from its critical function in crafting the lower ligand of vitamin B12, a molecule that underpins systems from nerve function to red blood cell formation. Pharmaceutical labs harness its framework to craft enzyme inhibitors and antifungal leads. Specialty dye companies have coaxed new pigments and colorants from its ring system for textiles and biomedical imaging. Analytical chemists often deploy it as a precise reagent in trace metal detection, offering low detection limits and sharp selectivity.
Research teams keep chasing sustainable alternatives to classic synthetic routes. Advanced catalysis, biocatalytic techniques, and renewable feedstocks have begun shifting pilot studies and early-stage commercial ventures to greener territory. Computational modeling and structure-activity studies use the benzimidazole nucleus to predict potential new drugs long before they're made in the lab, speeding up discovery timelines. One exciting thread involves coupling 5,6-dimethylbenzimidazole to metal centers for next-generation sensors, putting high-value analytics within reach for small labs and fieldwork.
Toxicologists continue to probe its safety, and evidence so far supports careful use in regulated settings. Acute oral and dermal studies in animal models show limited toxicity at practical concentrations, with few signs of major organ disruption or mutagenicity. Still, chronic exposure remains a concern. Repeated handling without protection may cause skin or respiratory irritation, urging operators toward consistent PPE use. Environmental fate studies indicate minimal persistence and a low risk of bioaccumulation. Ongoing surveillance and thorough documentation keep this data alive for everyone in the science chain.
With tailored catalysis, digital synthesis planning, and smarter process engineering entering the mix, 5,6-dimethylbenzimidazole looks set for wider roles well beyond its vitamin B12 origins. Cross-disciplinary teamwork between chemistry, environmental science, and biomedical fields keeps spawning new derivatives and uses. Markets for personalized nutrition, precision diagnostics, and advanced functional materials all present fresh opportunities for this well-characterized compound, and transparent regulatory models will help tap this potential safely and responsibly.
People run across scientific names every day and probably skip over them. 5,6-Dimethylbenzimidazole sounds complex, but its story weaves straight through the daily vitamin routines and the food on our tables. This compound forms a big piece in the complicated puzzle that is vitamin B12. For anyone like me who grew up worrying about iron and “B” vitamins thanks to grandparents who always made sure a diet pill was followed by a glass of fresh milk, vitamin B12 held a special spot. What most never realize: 5,6-Dimethylbenzimidazole acts as an anchor inside the actual vitamin molecule that allows the vitamin to do its work in the body.
Bacteria in the soil and even in our digestive system need to build vitamin B12 from scratch. 5,6-Dimethylbenzimidazole doesn’t show up in carrots or cheese by accident. It gets built by some clever bacteria, linking into the core of B12. This connection allows the entire vitamin to interact with enzymes and triggers the reactions that keep blood cells healthy and nerves firing properly. Studies like those out of the University of Cambridge have revealed that missing this component, the body does not absorb or use B12 well. That’s not just a test-tube finding. Vitamin B12 deficiency risks creep up fast without this tiny structure, from tiredness to memory loss.
In the vitamin supplement industry, companies aim for the most stable and reliable vitamin forms. 5,6-Dimethylbenzimidazole ends up synthesized in labs to make vitamin B12 on a commercial scale. Laboratories focus on this compound’s purity, since even small contaminants will make a difference in the quality of the final vitamin pills. Contamination or a breakdown in this structure impacts safety and the real benefits to people who rely on supplements, especially vegans, elderly folks, and those with stomach absorption issues.
Ingredients like 5,6-Dimethylbenzimidazole don’t exist in a vacuum. Production often relies on petrochemical feedstocks and resource-intensive synthesis methods. Waste generated during production can become a problem if not managed. Regulations in Europe, North America, and Asia set out rules to cut down pollution and increase transparency about what goes into supplements and animal feed. Still, the global demand for B12, especially in population centers shifting toward plant-based diets, means environmental challenges remain steady in the background.
Researchers keep digging into new ways to produce vitamin B12 using genetically-altered microbes that churn out more of the active molecules with less chemical mess. Better controls and certifications can push manufacturers to show every component’s source and limit environmental downsides. It helps to keep regulators, scientists, and consumer advocates involved in this process. For the everyday person picking up a bottle of multi-vitamins or deciding to supplement a diet for health reasons, knowing about the path from a laboratory compound like 5,6-Dimethylbenzimidazole right up to the final capsule gives a better idea of the science and ethics behind our nutrition choices.
Access to accurate, easy-to-understand information about these building blocks of vitamins, backed by peer-reviewed studies and expert oversight, helps everyone—parents planning meals, patients managing chronic illness, or everyday folks aiming for better health—make real, sound decisions. The science might sound obscure, but the ripple effects reach the breakfast table and the dinner plate, every day.
Across pharmaceutical labs and nutrition companies, 5,6-Dimethylbenzimidazole finds its way onto shelves and benchtops as a core building block for Vitamin B12 synthesis. Its presence in research and industry is not a random accident—it serves a meaningful role, but just like any chemical, respect for its potential hazards matters.
Most people don’t handle this compound every day. For those who do, gloves, goggles, and a fume hood aren’t just accessories—they’re basic armor. Even if you’ve logged hundreds of hours with fine white powders, the right safety routine should feel as natural as wearing a seatbelt. Published data shows 5,6-Dimethylbenzimidazole can irritate skin, eyes, and respiratory tract. Anyone weighing, mixing, or transferring this stuff holds the power to limit risks through simple, proven steps.
I’ve watched new lab techs let down their guard, leaning too close to flasks and forgetting the importance of good ventilation for small-scale syntheses. Those first moments of irritation remind everyone that safety protocol has real purpose. Researchers have reported mild but real symptoms with accidental inhalation. In some older studies, repeated exposure led to throat and nasal irritation, signaling the need for solid personal protective gear.
Industry documentation for 5,6-Dimethylbenzimidazole lists it as a chemical with low acute toxicity, and no concrete evidence ties it to long-term organ damage in humans. Yet the absence of a red-flag doesn’t mean carte blanche. Chronic low-level exposure to benzimidazole compounds—if ignored—has led to mild liver effects in animal studies. I’ve learned the importance of minimizing dust and working in well-ventilated areas. Never brushing off a safety alert keeps everyone a step ahead.
Unlike table salt, this powder shouldn’t sit unsealed on benches. Proper containment means storing 5,6-Dimethylbenzimidazole in air-tight containers, marked with hazard symbols. Too many spills start with a loose cap or careless weigh-out. Vacuuming up the mess isn’t recommended; sweeping with gentle movement and wearing a mask reduces particle dispersal and personal exposure. Even if a spill seems minor, approaching cleanup with gloves on is a habit worth keeping for the long haul.
Labeling is a glimpse into responsible handling. Hazard icons mean something. Reading manufacturer safety data, maintaining a spill kit, and familiarizing yourself with first-aid procedures all matter. A simple eye wash station, always within reach, often saves the day if powder or dust unexpectedly finds its target.
Routine makes safety simple. Wearing gloves—nitrile for most chemicals—keeps skin safe from sudden splashes or contact. Fume hoods protect against dust clouds, especially if weighing more than a gram or two. Lab coats and goggles add more layers. At home, proper disposal for chemical waste avoids environmental risks. Flushing powders down the sink might seem easy, but collecting waste in sealed containers protects both pipes and public waterways.
Teaching new team members about the quirks of benzimidazole derivatives pays off. Quick reviews of incident reports and clear communication shape a culture where people look out for one another. In the end, real safety around 5,6-Dimethylbenzimidazole depends on awareness, habit, and mutual support—much more than any warning label could cover.
Growing up around labs and later working in one, I learned early that storing chemicals isn’t something to take lightly. 5,6-Dimethylbenzimidazole pops up in many research circles. It’s a solid compound, important for vitamin B12 synthesis and other laboratory uses. Every scientist who’s opened a container knows: if you cut corners with chemical storage, you could risk everything from lost time to serious accidents.
Small molecules like 5,6-Dimethylbenzimidazole change and degrade when conditions aren’t just right. That leads to wasted resources, verifiable errors in your research, and sometimes, dangerous situations. For this compound in particular, research and safety data show it stays stable at room temperature, but air and moisture start to break it down. That powder loses punch once it picks up water from the air—clumping, changing color, or not reacting the way it should in your experiment.
I remember a shared storage room in grad school. Someone left a benzimidazole vial half-open. A week later, there was visible discoloration inside. Nobody trusted that sample afterwards. Contaminated or degraded stock doesn’t just cost a few dollars; it can set a project back weeks.
5,6-Dimethylbenzimidazole belongs with the rest of your solid organics—in a cool, dry place where sunlight can’t sneak in. That usually means a tightly sealed glass container, tucked in a cabinet that resists humidity. Many labs favor desiccators. Even if your lab doesn’t have fancy equipment, using silica gel pouches in a regular container helps soak up stray moisture.
Regular glass jars work fine for a week or two, but screw-cap bottles with an extra plastic seal prove worth the small extra cost. Light exposure speeds up decomposition. I once watched a sample fade after only a few days on a sunlit bench. Out of sight doesn’t just mean out of mind; in chemical storage, it also means extra weeks of shelf life.
The temperature factor gets overlooked. High temperatures, like those from radiators or windowsills, can start chemical changes in 5,6-Dimethylbenzimidazole. Every chemistry instructor I’ve known harps on the same rule: no organics near the window. Shelves in the center of the room, or in a controlled storage cabinet, keep everything better preserved.
My old supervisor told us: treat every bottle like it’s just as dangerous as the last, and your luck will hold. 5,6-Dimethylbenzimidazole isn’t the nastiest chemical out there, but inhaling its dust or getting it on your skin guarantees harsh irritation. Always handle with gloves and eye protection, and keep the cap on unless you’re weighing or diluting.
In my experience, safety data sheets collect dust on the shelf. Real safety means every new user gets a five-minute walkthrough. Show people the right spot on the shelf and how to close the lid tight. That’s more valuable than any warning sign.
Automation offers one path. Labs on a budget track sample inventory on spreadsheets. A modern approach uses barcodes or RFID chips to monitor opening dates and trigger reminders for periodic checks. Group training goes a long way to reinforce proper storage, and after-hours checks catch cracked seals or misplaced containers.
Anyone handling 5,6-Dimethylbenzimidazole wants it to last. Good storage isn’t just about saving money or time; it keeps people healthy and projects moving. Simple habits—cap bottles tightly, shield from light, lock away the container—prevent most problems from ever starting.
Every day, labs across the world work with compounds whose behavior can make or break a research project. In my student years, mixing up just a single atom in a formula meant blank stares from both my professor and my benchmate. The formula isn't just symbols—it's the starting line for every hypothesis, safety measure, and application. Without accuracy, the rest of the experiment crumbles. So, even before anyone talks about synthesis or uses, nailing down the right molecular formula matters big time.
Let’s talk structure. Benzimidazoles show up often in medicinal chemistry, and their structure—a fused benzene and imidazole ring—offers versatility. Now, add two methyl groups at positions 5 and 6. Now we're looking at a specific variant: 5,6-Dimethylbenzimidazole. Its molecular formula is C9H10N2. This might look simple, but each atom carries meaning. The two methyl groups send carbon and hydrogen counts up, while nitrogen stays at two, embedded in the ring system.
I remember working on a project where benzene derivatives kept coming up. Someone tried using a benzimidazole analog with a single methyl group instead of two. The effects on solubility and reactivity jolted the whole system. There’s a reason pharmaceutical companies check and double-check these numbers. Even a single extra hydrogen or carbon changes how molecules interact in the body. In vitamin B12 synthesis research, 5,6-dimethylbenzimidazole isn't just a bench curiosity—it’s the lower ligand. Studies published in the Journal of Biological Chemistry confirm the unique properties that the dual methyl groups bring, supporting electron transfers central in life processes.
Lab supply companies know quality depends on getting these formulas right—there’s too much at stake to cut corners. You don’t want to order benzimidazole and end up with 5,6-dimethylbenzimidazole by mistake, or the other way around. It’s happened before, and the wasted time can be brutal. Standard chemical data repositories like PubChem and ChemSpider—both respected by experts—list the formula as C9H10N2. That agreement among respected sources means scientists can trust they’re working with the right building blocks.
The best way to avoid slipups? Double-check sources. Reputable suppliers always provide not just the compound name, but also a full chemical structure and a precise molecular formula. Strong lab practices depend on this focus: using certified reference materials, authenticating compounds with NMR or mass spectrometry, and keeping digital inventory records. In my experience, these practical steps save time and avoid costly reruns of failed reactions. Thoughtful protocols, team discussions, and real-world quality controls form the bedrock of trustworthy results.
Whether the work is basic research, pharmaceutical development, or anything in between, 5,6-dimethylbenzimidazole’s details matter. Getting the molecular formula right—C9H10N2—opens doors for real progress and real discoveries. It’s the difference between theory and result, frustration and breakthrough.
5,6-Dimethylbenzimidazole shows up in scientific circles mostly as a precursor in vitamin B12 synthesis. Not too many people outside chemistry departments even know this compound exists. I remember running into it first during an undergraduate research project — we struggled to get a small sample just to test a method. Nearly every supplier required us to fill out forms, prove our affiliations, and sign agreements. Some thought chemistry was about mixing colored liquids, but getting hold of just a few grams of this stuff started feeling more bureaucratic than academic.
International rules mean you can’t just buy 5,6-Dimethylbenzimidazole off a regular e-commerce site. Chemicals like this end up restricted because they have uses in controlled industries, so legitimate vendors have to follow strict distribution protocols. Sigma-Aldrich, Thermo Fisher Scientific, and TCI America each require business verification, and buyers often need to show a research connection. Years back, a colleague tried to buy similar substances as an independent scholar and got denied. The block wasn’t personal; it centered around safety and compliance. Without controls, even lab chemicals could wind up in the wrong hands.
Suppliers usually don’t sell to individuals. Even for small research labs, purchase approval sometimes takes weeks. The chemical comes with hazards: it may cause harm if mishandled, and spills can quickly turn into trouble. The routes to buy always seem tedious until one reads about chemical accidents from improper storage or misuse. Regulations may look like hoops, but I’ve seen them save reputations, careers, and even lives. Chemical leaks in school labs or amateur settings easily jump from learning moments to emergencies.
Some online forums float offers of 5,6-Dimethylbenzimidazole with no paperwork. A curious person might get lured by lower prices or quick shipments, but fake sources run huge risks. I’ve talked to grad students tricked into buying chemicals from “gray market” sellers; products arrived mislabeled or contaminated, and a few barely avoided serious trouble with campus security. Working with unverified substances also wrecks experimental results. Professional science rests on clear records and trusted materials, not mystery powders from sketchy websites.
Researchers can advocate for better communication with suppliers and more efficient approval steps, but safety wins out every time. Partnerships between academic departments and chemical companies help — I’ve seen institutions create purchasing accounts that streamline paperwork. Some campuses form trusted groups or consortia and negotiate with suppliers for regular, compliant deliveries, easing the logjams. For solo inventors or amateur chemists, community access points through accredited makerspaces could offer supervised handling and group orders, always under strict protocols and safety training. What counts isn’t just ease, it’s the shared promise not to cut corners. Science thrives on trust, and chemicals like 5,6-Dimethylbenzimidazole remind me why this trust matters so much.

| Names | |
| Preferred IUPAC name | 5,6-Dimethyl-1H-benzimidazole |
| Other names |
5,6-Dimethyl-1H-benzimidazole 5,6-Dimethylbenzimidazol 5,6-Dimethyl-1,3-benzimidazole |
| Pronunciation | /ˌfaɪv.sɪks-daɪˈmɛθɪl-bɛnˌzɪmɪd.əˌzɔːl/ |
| Identifiers | |
| CAS Number | DIMETHYLBENZIMIDAZOLE |
| 3D model (JSmol) | CT1000265247 |
| Beilstein Reference | 120875 |
| ChEBI | CHEBI:16337 |
| ChEMBL | CHEMBL1230771 |
| ChemSpider | 16201 |
| DrugBank | DB02027 |
| ECHA InfoCard | 198915 |
| EC Number | 220-386-5 |
| Gmelin Reference | 81642 |
| KEGG | C06586 |
| MeSH | D003657 |
| PubChem CID | 70038 |
| RTECS number | UU7525000 |
| UNII | 2K1V7GP655 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID4020355 |
| Properties | |
| Chemical formula | C9H10N2 |
| Molar mass | molar mass: 146.19 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.14 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.91 |
| Vapor pressure | 0.00256 mmHg at 25°C |
| Acidity (pKa) | 5.51 |
| Basicity (pKb) | 2.86 |
| Magnetic susceptibility (χ) | -70.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.6200 |
| Viscosity | 1.34 cP (20°C) |
| Dipole moment | 1.89 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 218.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 97.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3776 kJ/mol |
| Pharmacology | |
| ATC code | N7XX |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H319 |
| Precautionary statements | P261, P264, P270, P271, P301+P312, P304+P340, P312, P330, P405, P501 |
| Flash point | 99°C |
| Autoignition temperature | 440 °C |
| Lethal dose or concentration | LD50 (rat, oral): 2730 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1770 mg/kg (oral, rat) |
| PEL (Permissible) | No specific PEL established. |
| REL (Recommended) | 5 mg |
| Related compounds | |
| Related compounds |
Benzimidazole 2-Methylbenzimidazole 4,5-Dimethylimidazole 4-Methylimidazole Imidazole |