Back in the late 20th century, chemists grew curious about the ways sulfur could influence amino acid scaffolds. Traditional proline derivatives caught plenty of attention, tracing their footsteps from peptide synthesis and asymmetric catalysis breakthroughs. Solid-phase synthesis owed its efficiency to such tweaks. As researchers probed further, the swap of a phenylsulfur group at the 4-position of L-proline set off a run of experiments that drew structural biologists, medicinal chemists, and reaction engineers. Now, (4S)-4-(Phenylsulfanyl)-L-Proline has staked its claim in libraries of small organic molecules valued for investigation.
At the core, (4S)-4-(Phenylsulfanyl)-L-Proline builds on the L-proline backbone. Add a phenyl group swinging from a sulfur at the number four carbon, and you get depth in reactivity and biological targeting. Labs see this molecule as a bridge—sitting between classic amino acids and next-gen pharmacophores. Its structure invites curiosity, promising more than the sum of its parts. Experience says its adaptability extends to both academic puzzles and process-scale manufacturing lines.
This pale crystalline solid doesn’t easily dissolve in water but prefers polar aprotic solvents like DMSO and DMF. It brings a molecular weight of roughly 239 grams per mole. The backbone reflects the defining proline ring, but with sulfur and phenyl additions, solubility and partition coefficients take a noticeable shift. Melting point edges up compared to unsubstituted proline, which suggests changes in crystal packing. In NMR spectra, aromatic protons stand out, and sulfur shifts make the signature unmistakable. High purity samples head out of commercial suppliers with over 98% HPLC quality, with spectral libraries growing year by year.
Reagent bottles get filled with a fine white or off-white crystalline powder. Labels name it by its IUPAC name—(2S,4S)-4-(phenylthio)pyrrolidine-2-carboxylic acid—or several synonyms, so informed buyers always double-check catalog numbers and chemical structures. Certificate of analysis lists batch number, purity percentage, analytical method, and water content. Safety data sheets accompany every shipment, outlining stability under shelf conditions, flash point, and incompatibilities. Barcode systems link bottles with ordering and stock systems for traceability in regulated environments. Users scan to access all technical bulletins and change notifications.
One practical route starts with L-proline, activating the 4-position through halogenation steps—but only after protecting the amine and carboxyl groups. Nucleophilic substitution with thiophenol delivers the phenylsulfanyl moiety. Reaction times hinge on temperature, base, and reagent grades. After coupling, deprotection releases the target product, followed by rigorous chromatographic purification. Side products, like disulfides or over-substituted species, demand patience for full separation. Scale-up calls for solvent recycling, in-line analytics, and careful waste stream management to comply with environmental expectations.
Chemists treat this proline derivative as a springboard. The sulfur-pheny group accepts oxidation to sulfoxide or sulfone, unlocking new reactivity and biological properties. Peptide synthesis benefits from deploying this motif at critical positions, changing protein folding and enzymatic activity in test runs. The carboxyl group enables coupling for peptide or amide bond formation, while the amine supports derivatization, including Fmoc or Boc protection for solid-phase synthesis. Some groups probe Suzuki or Heck reactions on the aromatic ring, customizing the phenyl’s electronics for structure–activity relationship studies. Analytical follow-up with LCMS and NMR keeps modifications grounded in solid data.
This compound hides behind many names across catalogues and papers. Some write it as (2S,4S)-4-(phenylthio)proline, others use its systematic moniker. Catalog numbers shift with each supplier—Chemscene, AK Scientific, and BLD Pharm each have entries. Internally, project teams sometimes reference shorthand abbreviations or codes, so researchers often cross-verify names against structural diagrams to prevent mix-ups. International databases collate synonyms, easing global procurement and regulatory navigation.
Lab safety officers instruct on handling—avoid inhalation, skin contact, and wear gloves plus safety goggles. SDS sheets classify the solid as irritant category, calling for local exhaust ventilation if handling on a larger scale. Waste solvents and washings get collected in labeled drums, awaiting compliant disposal. Emergency protocols highlight rapid response steps in case of accidental exposure. Flammable and corrosive reagents used in synthesis also require segregation. Larger facilities fit storage areas with climate and humidity controls, routine shelf-life monitoring, and fire suppression gear. Regulatory teams ready all compliance documents for audits, a regular feature on the path to ISO or GMP certification.
Pharmaceutical research teams reach for (4S)-4-(Phenylsulfanyl)-L-Proline to break new ground in peptide drug discovery and enzyme inhibitor design. Structural features open doors to chiral catalyst design, giving fine control over reaction outcomes. Bioorganic chemists swap proline for this derivative in peptide loops, testing for shifts in secondary structure and receptor affinity. Material science applications surface in areas demanding stability and redox-switchable motifs. Education labs sometimes use it to illustrate how simple structural tweaks reshape molecular behavior. Its presence in chemical libraries supports docking studies and structure–activity screening on virtual and physical platforms.
R&D groups explore analog synthesis, working through structural variations on the phenyl ring. Combinatorial chemistry platforms plug in the core structure, broadening property screens. Some alliances pair this compound with proprietary detection tags, mapping protein–protein interaction sites or enzyme substrates. Funded projects in academic labs chase the development of new peptidomimetics for tackling antibiotic resistance and metabolic diseases. Conferences fill up with posters outlining recent results—each iteration refining process, performance, and application fit. Computational studies back up experimental campaigns, offering molecular modeling perspectives unavailable just a decade ago.
Toxicology departments add this proline derivative to in vitro and in vivo studies as part of early-stage drug safety screening. Cell cultures get exposed in dose-response experiments, looking for signs of cytotoxicity or altered proliferation patterns. Animal studies, more tightly regulated, scan for organ-specific effects and metabolic breakdown products. Analytical chemists track metabolites across sample matrices, hunting for stability and unwanted accumulation. Lessons from sulfanyl-containing pharmaceuticals guide focus on potential allergic responses or sulfur-based toxicities, while close collaboration keeps between medicinal chemistry and safety teams. Results shape dosing limits, handling guides, and regulatory filings.
Industry insiders see the utility of (4S)-4-(Phenylsulfanyl)-L-Proline growing. Rapid advances in peptide drug design and asymmetric synthesis mean chemists hunt for building blocks with distinct reactivity profiles. Start-ups and established companies alike invest in tailored amino acid derivatives, seeking patent space and first-mover advantages in therapeutic pipeline development. Greater access to automated synthesis tools lowers barriers for academic and industrial users. As green chemistry frameworks expand, emphasis lands not just on reactivity but on process safety and lifecycle impact, pushing for manufacturing upgrades. Regulatory clarity continues to rise with more widespread use and safety profiling. Globalized sourcing and digital lab management systems help keep research seamless. Looking out a few years, this molecule likely winds up in toolkits across pharmaceutical, chemical, and materials science sectors—each finding new ways to press sulfur-phenyl linked proline toward fresh frontiers.
Organic chemistry students and working scientists alike bump into complicated molecules all the time, but some of these structures just jump off the page. (4S)-4-(Phenylsulfanyl)-L-Proline draws attention, both for its tongue-twister name and for its unique chemical backbone. At first glance, it may look like another substituted amino acid, but as you break it down, this molecule brings some chemistry lessons to life.
Proline stands out from other proteinogenic amino acids because its side chain bonds to the amino group, forming a ring. Take that foundation and change the fourth carbon—on the “S” (for sinister or left-handed) chirality—by swapping a typical hydrogen atom with a phenylthio group. That means you tack on a benzene ring, connected by a sulfur atom, replacing a small, unassuming hydrogen with a bulky and aromatic group. Chemists write its skeleton as C11H13NO2S. Its backbone looks like this: a five-membered pyrrolidine ring holding the amino and carboxyl groups, with the game-changing substitution sticking out at carbon 4, locked in the left-handed orientation.
The introduction of the phenylsulfanyl group at the fourth position does more than dress up proline; it changes both physical and chemical properties. This kind of substitution expands the molecule’s reach—now it's not just involved in the biology of proteins but also steps into pharmaceutical research, peptide design, and enzyme mechanisms. The sulfur link, often called a “sulfanyl” or “thioether,” brings in reactivity and can even tweak how the molecule interacts with biological targets.
Researchers often use substituted proline derivatives as building blocks while designing drugs or materials because small changes at key locations can totally reshape a compound’s behavior. In this molecule, the phenyl ring introduces aromatic stacking potential, meaning it can interact with other aromatic compounds and proteins in ways plain proline never could. The sulfur acts as a flexible bridge, and I’ve found it often creates possibilities for further reactions or even light sensitivity.
Scientists love to take inspiration from nature, but most biological systems don’t feature this sort of proline. Still, chemists apply these modifications to create enzyme inhibitors or peptides with improved stability and bioavailability. The bulkiness from the phenyl ring can block enzymes or receptors, nudging reactions down specific pathways. Adding sulfur sometimes helps drugs cross membranes or dodge certain metabolic roadblocks. In peptide drug research, non-standard amino acids like this see trials as tools to resist breakdown in the body, meaning they could play a role in medicines that work longer or hit their targets more reliably.
Making (4S)-4-(Phenylsulfanyl)-L-Proline brings its own challenges. Controlling the specific stereochemistry at the fourth position isn't just picky chemistry—it’s essential for getting results that match biological systems. A wrong-handed version (the “R” isomer) might fail or behave unpredictably. Having spent time troubleshooting amino acid syntheses, I know how a single misstep can leave you with an inactive or even toxic product.
Exploring molecules like (4S)-4-(Phenylsulfanyl)-L-Proline pushes the boundaries of what chemists can do with amino acids. Every tweak in the structure opens up new lines of investigation for drug design and molecular engineering. With careful structure control and smart synthetic choices, derivatives like this could support the next wave of precision medicine or offer clues into how proteins function when nature goes off script.
I’ve seen how the pharmaceutical world loves a good challenge, especially one solved by a clever molecule. (4S)-4-(Phenylsulfanyl)-L-Proline caught my eye in research labs pretty often because chemists appreciate what this proline derivative brings as a chiral building block. Drug development depends on chiral molecules to ensure medications work the way our bodies expect. The (4S)-enantiomer offers high selectivity, which helps in making treatments safer and more effective. It’s not just a stand-alone compound, either. Medicinal chemists use it to piece together larger, more complex structures that show promise as antivirals, antibiotics, or anti-cancer agents. Consider that many peptide-based drugs mimic how our own bodies work—adding a unique proline analogue can twist a peptide in just the right way to hit a tough target, or resist enzymes that would normally break drugs down.
Chemists have long turned to (4S)-4-(Phenylsulfanyl)-L-Proline as a sturdy foundation for custom molecules. The sulfanyl substituent on proline opens up new chemistry routes. For example, in asymmetric synthesis, small changes in building blocks like this can flip outcomes and make reactions work cleaner—this saves time and money in large-scale manufacturing. In my conversations with researchers, many mention this compound’s handy thioether group, which makes it possible to link up other pieces or tweak reactivity exactly where it’s needed.
A peptide is only as good as its side chains. Proteins depend on proline for turns and twists. Swapping in (4S)-4-(Phenylsulfanyl)-L-Proline alters how peptides fold—scientists experiment with such changes when designing inhibitors, enzyme mimics, or “smart” biological tools. It’s been used in developing new peptide ligands that stick tightly to disease targets. Some work even shows that using this analogue increases the stability of peptides in harsh biological environments, so drugs last longer in the body.
Applications stretch into supramolecular chemistry, too. Builders of “smart” materials seek out new amino acids to change how gels, films, or molecular assemblies behave. Adding phenylsulfanyl groups can help form gels that respond to outside triggers, like light or pH. While this might not grab as many headlines as cancer drugs, it’s big news for biosensors or carriers for slow-release medicines.
Supply chain worries always come up with specialized amino acids. Researchers try to streamline syntheses—greener chemistry and efficient recycling steps are making this molecule more available for academic and industrial projects. The more accessible (4S)-4-(Phenylsulfanyl)-L-Proline becomes, the more labs can dig into its quirks and strengths.
As new diseases emerge and old ones fight back, drug designers can’t ignore the edge a unique building block offers. By investing in innovative proline derivatives, the entire field gains tools to beat tough biological problems and drive progress, whether the setting is a patient’s bedside or a pilot plant bench.
Anyone who has spent time at a lab bench knows the frustration caused by unreliable materials. A single impurity can throw off a synthesis, ruin biological assays, or stall a project for weeks. With building blocks like (4S)-4-(Phenylsulfanyl)-L-Proline, which often plays a role in peptide chemistry and medicinal research, purity isn't just a luxury—it's a foundation.
Most commercial suppliers offer this compound at purities typically above 97%. That number matters. For comparison, pharmaceutical research projects usually reject anything under 95% purity for key intermediates, especially during lead optimization. This compound, provided at such high purity, fits right into research settings that treat each variable as critical.
Purity alone isn’t the whole story. The grade paints another layer of detail. When researchers order (4S)-4-(Phenylsulfanyl)-L-Proline, they look for designations like “research grade” or “analytical grade.” Analytical grade stands up to the strictest tests for contaminants, including heavy metals and residual solvents. Research grade, with slightly relaxed criteria, supports exploratory projects and early-stage development. Both grades usually guarantee traceability, batch certificates, and detailed spectral data.
I’ve worked on projects where skipping over grade documentation caused major headaches. Unknown contaminants interfere with mass spectrometry, and unexpected byproducts turn up during purification. High-grade starting materials help build trust in the data.
Solid suppliers provide certificates of analysis (CoAs) for every batch—these sheets are more than paperwork. They lay out exactly what was measured for each lot: water content, enantiomeric excess, residual solvents, and often NMR traces. It’s not just about listing numbers. The real work starts by digging into the details. A 98% pure sample, by HPLC, still might have unknown impurities unless the supplier discloses what those impurities could be.
Regulatory agencies have been tightening their standards. The FDA and EMA expect thorough documentation on every reagent used, not just finished products. So, every time a lab purchases a bottle of (4S)-4-(Phenylsulfanyl)-L-Proline, it should demand detailed analysis, not just a single purity percentage.
High-purity chemicals improve confidence in experimental data. Standardizing quality across suppliers makes a difference. It’s helpful to work with vendors who don’t just sell materials but help interpret spectral data and respond quickly to questions about a batch. Some suppliers even provide digital spectra for independent verification.
On the user end, don’t settle for ambiguity. Request certificates, ask for clarification, and reject bottles missing data. Internal controls—running a quick NMR or preparing an HPLC trace—cost time but save frustration and money in the long run.
The world expects reproducible science. (4S)-4-(Phenylsulfanyl)-L-Proline is just one piece, but it highlights a big principle: transparent reporting builds stronger results. Purity and grade are not just box-ticking exercises—they create the backbone of serious research.
Working in labs where chemical stability matters sharpens your senses. I’ve handled my share of sensitive compounds, and a thoughtful approach makes the job safer. (4S)-4-(Phenylsulfanyl)-L-Proline, with its unique side chain, fits in that tier of chemicals that demand respect from the moment the bottle arrives.
I’ve seen more than one experiment thrown off by a few stray water molecules. Even a hint of moisture can turn some chemicals into mystery substances overnight. The same goes for (4S)-4-(Phenylsulfanyl)-L-Proline. This compound won’t forgive careless sealing or a loose cap. Air, especially humid lab air, can slowly degrade sensitive organosulfur compounds like this one. In my experience, freshly packing the powder into an amber glass vial, squeezing out air with a stream of nitrogen, then screwing down a tight cap sets you up for success.
Years ago, my graduate mentor drilled into me: “room temperature means different things in different countries.” In the case of (4S)-4-(Phenylsulfanyl)-L-Proline, cooler is better. I keep mine in the fridge at +2°C to +8°C. This slows down any slow background reactions, which you might never notice until purity tests fail. Freezing between -15°C and -25°C can take shelf life from a year to over two, based on long-term studies of similar proline derivatives. If you’ve ever heard the rattle of glass vials in a frost-filled freezer during a late-night sample run, you know how common this best practice feels in real life.
Light exposes a subtle risk. Photodegradation creeps up especially with sulfur-containing moieties present, and the phenylsulfanyl group amplifies that worry. Amber glass or aluminum foil wraps have saved plenty of samples from yellowing or breakdown. Properly stored, the white to off-white powder stays that way. The difference shows up on purity readings, and sometimes just by eye. Chemical intuition tells me that light control buys you insurance against a host of unpredictable decomposition pathways.
Good scientists sweat the small stuff—few habits matter more than strong labeling and tracking. I clearly write the date received, date opened, and initial purity right on the vial. Simple, bold labeling stops mix-ups and answers the inevitable “how old is this batch?” question. Electronic inventory helps, but the label sits where you need it, every time you grab the sample. Consistency here makes teams stronger and catches storage mistakes before they threaten an experiment.
Cross-contamination turns research from hard to impossible. Using clean spatulas and keeping original packaging in a secondary sealed bag stops most stray dust or transfer. With (4S)-4-(Phenylsulfanyl)-L-Proline, you’ll notice very fast if a sample absorbs odors or catches on with organics left on benches or gloves. I keep gloves fresh and tools separate for each work session. This habit takes a few extra seconds and prevents so many future headaches, especially during small-scale synthesis or pharma R&D.
Proper storage of this compound relies on basics: cool temps, dry air, darkness, clear labeling, and clean technique. Each lab’s specifics shape the details, but these routines turn risks into reliability. The payoff arrives every time a reaction runs with predictable outcomes, every time you avoid strange smells or mystery degradation, and every time you waste less by pushing shelf life to the fullest. Consistent discipline with materials like (4S)-4-(Phenylsulfanyl)-L-Proline safeguards your work and, by extension, your reputation as a careful, capable chemist.
Anybody working in pharmaceuticals, specialty chemicals, or peptide research will hit a familiar hurdle now and then: sourcing the starting materials in quantities big enough to meet project needs. (4S)-4-(Phenylsulfanyl)-L-Proline, not exactly a household name, gets tossed into the spotlight here. It's a proline derivative, pretty useful when you’re after that sulfur-containing twist in a molecule, but not stocked by most chemical distributors in large drums. If you poke around, it becomes clear—most suppliers, big or small, treat this compound like a boutique item. Order a few grams, and you might get a fair price. Ask for kilos and that's another story.
In pharma R&D and scale-up projects, it’s not uncommon to burn through dozens or even hundreds of grams of a building block just for early-stage work—never mind what a pilot plant might request. Without a steady, affordable supply, a project stalls. Price tags balloon, timelines get stretched, and the bench chemist spends more time convincing accountants than actually doing chemistry. This has a knock-on effect: promising compounds die in development, not for lack of scientific potential, but from the uncertainty in sourcing.
Proline derivatives don’t just leap off catalog pages in bulk, and there’s a reason. Their synthesis takes finesse, especially once you tack a phenylsulfanyl group in the 4-position. Many commercial suppliers make to order and rarely keep kilogram quantities on hand. Each batch can vary a little in impurity profile, and scale-up can introduce new wrinkles. Customs red tape, shelf-life concerns, and handling regulations add layers of drama, especially for shipments moving across borders.
My own experience echoes what many chemists dealing with rare amino acids say. It’s common to rely on a small handful of specialized suppliers or even reach out directly to contract manufacturers. Some growers in India or China can pull off multi-step synthesis and ramp up quickly if you’re OK fronting a larger order and waiting out the lead time. Established names in fine chemicals like Sigma-Aldrich or TCI might offer a quote for 25g here or there, but it’s not quite the same as real bulk access. Contact enough companies, and you’ll notice smaller labs sometimes quote sky-high prices for what looks like a custom synthesis, while some larger players just send a ‘not available’ reply.
If manufacturers and consumers want better access, conversations need to open up. Genuine demand signals help smaller producers justify investment in scale-up. Buyers can pool orders where sensible, smoothing out unpredictable requests. Engaging with a reliable CRO or CDMO (contract development and manufacturing organization) who understands your urgency makes a big difference. Keeping supply chains robust and starting discussions earlier in the R&D process gives everybody a fighting chance against costly delays. In the meantime, researchers stay ready to pivot or tweak methods, knowing that supply-side hiccups are part of the journey.
Looking around today, you won’t see (4S)-4-(Phenylsulfanyl)-L-Proline lining warehouse shelves in 10 kilo bags, unless you’re lucky enough to have convinced a producer to accept your business case. Until then, many labs piece together shipments and regularly reassess trusted partners. Everyone in the industry knows the value of solid relationships, transparency in communication, and a willingness to negotiate for the compounds that keep innovation moving.
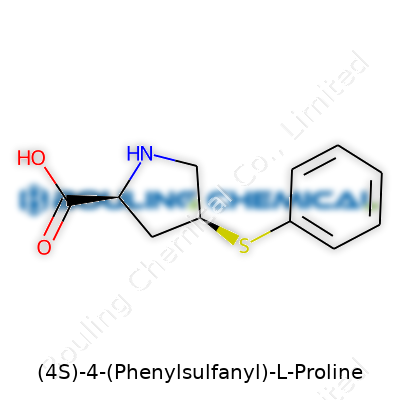
| Names | |
| Preferred IUPAC name | (2S,4S)-4-(Phenylsulfanyl)pyrrolidine-2-carboxylic acid |
| Other names |
(2S,4S)-4-(Phenylthio)proline (4S)-4-(Phenylsulfanyl)-L-proline ZygaPro (S)-4-Phenylthio-L-proline |
| Pronunciation | /ˈfɛnɪlˌsʌlˌfaɪl ɛl ˈproʊliːn/ |
| Identifiers | |
| CAS Number | 459166-07-9 |
| Beilstein Reference | 104194 |
| ChEBI | CHEBI:131435 |
| ChEMBL | CHEMBL1229640 |
| ChemSpider | 22211618 |
| DrugBank | DB08339 |
| ECHA InfoCard | 03b1c3f5-e1d3-43d7-8e31-38442e330f39 |
| EC Number | 873837-24-0 |
| Gmelin Reference | 110766 |
| KEGG | C18799 |
| MeSH | D064370 |
| PubChem CID | 5283622 |
| RTECS number | SY8575000 |
| UNII | I4003D7Z3Z |
| UN number | Not assigned |
| CompTox Dashboard (EPA) | DTXSID30969966 |
| Properties | |
| Chemical formula | C11H13NO2S |
| Molar mass | 275.36 |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.3 g/cm³ |
| Solubility in water | Insoluble in water |
| log P | -1.1 |
| Acidity (pKa) | 2.10 |
| Basicity (pKb) | 2.96 |
| Magnetic susceptibility (χ) | -78.64·10⁻⁶ cm³/mol |
| Dipole moment | 6.10 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 99.2 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | H315, H319, H335 |
| GHS labelling | GHS02, GHS07 |
| Pictograms | `CC(C1CC(NC1)C2=CC=CC=C2S)N` |
| Signal word | Warning |
| Hazard statements | No hazard statements. |
| Precautionary statements | Precautionary statements: P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P321, P332+P313, P337+P313, P362+P364, P501 |
| PEL (Permissible) | NIOSH: Not established |
| REL (Recommended) | 1.00 |
| Related compounds | |
| Related compounds |
L-Proline Proline 4-Hydroxy-L-proline cis-4-Hydroxy-L-proline trans-4-Hydroxy-L-proline |