Back in the era of growing silicone chemistry, practical research demanded materials that could bridge the gap between organic and inorganic worlds. As industries looked for compounds that allowed materials like glass or metals to “talk” to polymers, researchers set their eyes on organosilane pioneers. The morpholine derivative with a triethoxysilyl group emerged through experiments combining amino functional groups and reactive silanes. By the late 20th century, this molecule gained recognition in laboratories seeking powerful ways to modify surface activity, especially with an eye for durability and tailored reactivity. Initial reports showed that it outperformed earlier, simpler alkoxysilanes in terms of binding strength and compatibility with various substrates. Decades later, this compound has become a mainstay—engineers and chemists alike have asked for it under different names, but always with high expectations for performance and versatility.
This molecule straddles two worlds. On one end, the morpholine ring offers calm, persistent chemical stability, and on the other, three ethoxy groups linked to silicon practically beg for hydrolysis and subsequent bonding with silica-rich surfaces. The product usually shows up as a clear or nearly clear liquid, often stored in tightly sealed amber bottles to keep out moisture. It doesn’t take much in the way of lab expertise to realize that moisture in the air can start hydrolyzing those ethoxy groups, triggering premature condensation. I recall several cases where opening a bottle in a humid room led to a sticky mess forming at the rim, so proper storage makes all the difference. Chemists expect the sharp but not overpowering smell, typical of many morpholine derivatives, which quickly tells you you're working with an active, ready-to-react silane.
4-((Triethoxysilyl)methyl)morpholine features a molecular weight hovering close to 291.45 g/mol. Its refractive index often measures between 1.420–1.430, giving it a distinct signature on quality checks. Its boiling point pushes past 290°C, though practical work rarely needs such temperatures. The liquid mixes well with most common organic solvents like toluene and ethanol, making it easy to include in both lab-scale and industrial solutions. This chemical’s hydrolyzable ethoxy groups drive its surface action, splitting off ethanol under the right conditions and allowing the silanol formed to condense onto surfaces such as glass, quartz, or even metals treated with a thin oxide layer. In practice, I always watch for that slight cloudiness in solution as hydrolysis begins—a sign things are about to happen.
Suppliers typically label this compound by purity, which usually ranges from 97%–99%. Labels also identify hazards under GHS, pointing out the flammability and irritation risks that come with skin or eye contact. Shipping containers need proper UN numbers due to the blend of flammable ethanol produced by hydrolysis and the irritant properties of the morpholine ring. Labels mention its CAS number: 34762-90-8, a detail that brings up a long list of regulatory registration documents across the globe. Lot numbers and batch certificates help guarantee traceability, a principle that professional labs and regulatory bodies emphasize for any chemical involving human or environmental exposure.
Manufacturers usually produce 4-((triethoxysilyl)methyl)morpholine through a hydrosilylation reaction. This process brings together morpholine, a substrate primed with a reactive group, and the triethoxysilyl methyl group, often via a platinum-catalyzed addition to a vinyl or similar unsaturated group. The choice of catalyst, temperature, and solvent greatly influences yield and purity. I’ve seen cases where skipping careful purification by vacuum distillation led to stubborn yellow residues—those impurities can play havoc later by producing unpredictable curing profiles or unwanted byproducts in coatings. To avoid surprises, chemists verify every batch with NMR and IR spectroscopy, scanning for signals corresponding to morpholine hydrogens and the characteristic Si-O bonds.
Three ethoxy groups attached to the silicon atom act as the chemical “soft spot.” On contact with water, they hydrolyze, forming silanol groups, which then condense onto –OH rich surfaces, building durable Si-O-Si networks. This process underpins surface treatments for glass fibers and fillers. The morpholine end interacts with a wide range of polymers and resins, including epoxies and urethanes, by acting as a chemical crosslinker or compatibilizer. Modifying this molecule targets the morpholine or ethoxysilyl side to tweak solubility or reactivity. For example, swapping out the ethoxy groups for methoxy or propoxy versions affects how rapidly hydrolysis occurs in final formulations. Researchers seeking slower curing on surfaces might lean toward those alternatives. Direct chemical modifications of the morpholine ring are rarer, due to its stability and role in final performance, but I’ve seen patents suggesting alkyl extensions or substitution to modulate the interaction strength with specific resins.
Over the years, this compound has gathered a basket of alternative names. Common ones include: Morpholine, 4-(triethoxysilylmethyl)-; 4-(Triethoxysilylmethyl)morpholine; 1-(Triethoxysilylmethyl)morpholine; and sometimes “Silane coupling agent 3442” in some European catalogs. Keeping an organized list of these synonyms saves time searching safety data sheets or regulatory documents, especially in global supply chains where translations or regional nomenclature confuse things.
Working with 4-((triethoxysilyl)methyl)morpholine demands respect for its irritant properties. Direct skin contact brings risks of redness or burns, notably in humid conditions where hydrolysis speeds up. Ventilation helps by quickly moving away vapors, particularly during mixing or application steps in coatings. Eye protection and gloves remain standard practice, not just for compliance but because even tiny droplets find their mark during pipetting or transfer. Local exhaust ventilation and fume hoods cut exposure risks in both labs and factories. Safety data sheets set exposure limits, which reflect the combined hazards of both the morpholine and trialkoxysilane functionality. Spills on work benches rapidly build sticky, hard-to-scrub residues, making cleanup difficult unless solvents like ethanol are close at hand. Proper training for interns and staff keeps accidents low—routine drills and visible reminders matter more than once-a-year compliance box checks.
This compound has reshaped how industries prepare surfaces for bonding, painting, or coating. Glass manufacturers rely on it for prepping fibers before embedding them in composite plastics, boosting adhesion that survives years of mechanical stress. In electronics, printed circuit boards see improved conformal coatings by briefly treating surfaces with diluted solutions of this silane. Automotive plastics, often painted or laminated, resist peeling when pretreated with 4-((triethoxysilyl)methyl)morpholine. I’ve watched floor finishers in building construction use it in hybrid polymer sealants, where it binds mineral surfaces, like tiles or stone, to flexible resins. Many research groups also experiment with this molecule as a primer in nano-material synthesis or in developing advanced membranes for filtration. Its dual reactivity opens routes to new biomaterials or drug delivery coatings, combining stable surface attachment with compatible polymer chemistry.
Current R&D around this molecule mainly explores smart surface design and advanced hybrid materials. Teams try grafting it onto nanoparticles, expecting improved control in assembling thin films or membranes that filter water or gases. Polymer engineers use it in trial blends, searching for improvements in compatibility and toughness, especially in composite or multi-phase materials. In the context of modern electronics, the drive for ever smaller, stronger components has sent researchers sprinting back to the drawing board with organosilanes, 4-((triethoxysilyl)methyl)morpholine among the chosen few. Academic studies now model reaction kinetics and diffusion profiles, since fine-tuning when and how hydrolysis proceeds directly influences mechanical and chemical stability in real-world applications. Some research groups also revisit aging mechanisms, particularly how environmental humidity and UV light affect covalent bonds over long periods—knowledge critical for applications in aerospace and outdoor infrastructure.
Past studies on the toxicity of 4-((triethoxysilyl)methyl)morpholine examine both immediate and long-term exposure effects. Short-term research points to moderate irritation for eyes and skin, a hazard compounded in humid environments due to rapid hydrolysis. Animal studies track the compound’s metabolic fate and show low levels of bioaccumulation, which helps shape workplace exposure limits. Chronic exposure studies remain sparse, pushing regulators and manufacturers to advise extra caution, particularly in poorly ventilated work conditions. No conclusive evidence suggests high carcinogenicity, but the morpholine ring’s structural similarity to related amines sends up alert flags for health and safety committees. Responsible use includes routine air monitoring in production lines and mandatory spill response training. On the environmental side, breakdown products slow their way through soil and water, raising concerns about accumulation if handling becomes careless on a large scale. Industry guidelines increasingly call for closed-loop waste systems and careful effluent treatment.
4-((Triethoxysilyl)methyl)morpholine has potential to drive new advances in surface and polymer science. As demand grows for greener, more durable composites and hybrid materials, this compound stands at the intersection of stability and surface chemistry. Future research may focus on biocompatible modifications, opening paths for the compound’s use in drug delivery or implant materials. More eco-friendly synthesis routes are under development, aiming to trim energy usage and reduce hazardous waste in the production chain. As digital manufacturing technologies like 3D printing expand into new materials, 4-((triethoxysilyl)methyl)morpholine helps unlock printing of parts with custom-tuned, surface-bound functionality. In the coming years, regulations will tighten around toxicity, pushing for more transparency and sustainable alternatives, but the strong reputation of this molecule—built from decades of reliable performance—will keep it as a central player in the next phase of material science.
Picture repainting a room just to watch chunks of color peel off within months. That painful scene happens when surfaces and coatings don’t grip. 4-((Triethoxysilyl)Methyl)Morpholine often stands behind longer-lasting paints and sealants, helping molecules get a firm grasp on glass, metal, or concrete. This chemical forms a bridge between inorganic surfaces and organic coatings. It holds everything together—no gunky build-up or uneven patches. Chemists like it because it locks onto minerals on one end, and holds paint resin on the other. Reliable adhesion saves time, money, and plenty of headaches for building owners and maintenance teams.
Stored lumber or concrete, left untreated, soaks up moisture like a sponge, leading to mold, crumbling, or corrosion. Once 4-((Triethoxysilyl)Methyl)Morpholine enters the mix, it provides a set of water-repelling hands that keep interiors dry. Moisture protection isn’t just about comfort—it directly affects the longevity and safety of infrastructure. Researchers have shown that treating surfaces with silane-based chemicals cuts down on water incursion. In real life, this means fewer repairs and lower risk of dangerous mold blooms or concrete rot.
Modern medicine uses implants, tubing, or devices that need chemical stability and reliable surfaces. Manufacturers apply this chemical to modify glass or metal, improving biocompatibility for tiny parts inside human bodies. By tweaking the outer layer of a material, it reduces unwanted reactions or bacteria growth. Hospital staff and patients benefit from sterile instruments and longer-lasting devices. Scientific studies from Springer and ACS point out this chemical’s role in making laboratory glassware resistant to residue and easy to clean. Livelier, safer labs come with fewer errors, and that benefits everyone, from surgeons to patients.
Engineers search for ways to make computer chips and screens strong as well as reliable. Moisture, heat, and static can turn a shiny new phone into a dead weight in your pocket. The morpholine part of this compound gives electronics better surface strength and blocks moisture while causing no harm to tiny circuits underneath. In manufacturing, tech companies use silane modifiers so that touchscreens resist fingerprints, stains, and static. That’s not just about looks; lasting protection reduces e-waste and lets consumers hold onto devices longer.
Factories, breweries, or food processing plants rely on equipment that resists both chemical spills and regular scrub-downs. 4-((Triethoxysilyl)Methyl)Morpholine toughens up floors, pipes, and walls so that grime doesn’t stick and cleaning is faster. A research article from the Journal of Coatings Technology and Research confirms improved stain resistance and reduced cleaning costs after treatment. That stacks up to a safer workplace and a healthier bottom line.
There’s room for improvement. Environmental groups and sustainable tech innovators have started asking about impact throughout a product’s life. Chemists are tweaking recipes to keep environmental risk low and recycling simple. More transparent data-sharing between chemical makers, researchers, and regulatory agencies would push the industry toward safer practices. Reliable sources, including regulatory reviews, support ongoing safety tests and green chemistry efforts, ensuring that this helpful chemical fits into a safer, longer-lasting future.
Working in a lab brings daily reminders about chemical safety. 4-((Triethoxysilyl)Methyl)Morpholine doesn’t just sound complex—it actually is. Used in coatings or advanced materials work, it’s a specialty silane that handles potential crosslinking with care. Anyone who’s uncapped these reagents knows how rapidly they run afoul with moisture and high temps. Keeping them stable means following some rules that make a real difference.
Heat does bad things to organosilanes. I’ve seen older bottles turn cloudy after sitting too close to a steam radiator or left in an unventilated storeroom through the summer. Vendors like Sigma-Aldrich set a baseline: keep it under 30°C, ideally closer to 15–25°C. That fits a normal chemical storeroom, but direct sunlight or a spot near the ceiling lights can cause silent headaches.
Humidity ruins these chemicals faster than most people realize. 4-((Triethoxysilyl)Methyl)Morpholine reacts with water in the air, producing unwanted by-products and cutting its shelf life. Standard practice is to store bottles tightly closed and add packets of desiccant. Sometimes folks in the lab skip this step, but sooner or later, an expensive bottle will show up as a gummy mess.
I store my silanes in their original, well-sealed containers. Check the caps every time. If using a lot, look into a glovebox or at least a dry cabinet—those protect big investments. In smaller academic labs, I usually toss in a silica gel pouch. It’s cheap insurance. Avoid plastic bags, since leaks and poor sealing let water vapor creep in.
If you have more than one silane-based compound, separating storage keeps things safer. Some labs keep clear labels on secondary containment, listing the chemical name, hazards, and last date of opening. Don’t store next to acids or bases, which can accelerate degradation. Organic solvents sitting nearby often mean trouble after accidental spills. I keep all my alkoxysilanes grouped on a dedicated shelf, away from oxidizers and open beakers of water.
Small bottles make tracking use much easier. At my last lab, we labeled every new bottle with the date received and opened. Older stock moved to the front of the cabinet, so nothing sat forgotten and decomposed. It turns out expiration dates mean something—especially for a chemical that transforms with moisture or air. If in doubt, run a quick test for clarity or assess viscosity compared to a fresh batch. The nose often knows—sour or sharp odors mean the chemistry changed for the worse.
Mistakes happen. Spills of undiluted 4-((Triethoxysilyl)Methyl)Morpholine need careful cleanup with absorbent material, all done while wearing nitrile gloves and a face shield or goggles. Don’t pour unused stocks down the drain—check with hazardous waste collection for proper destruction instructions. Good housekeeping here prevents minor issues from turning into lab shutdowns.
Lab safety never feels glamorous. Most times, storage rules seem like common sense—keep cool, dry, and tightly sealed. Still, cutting corners on chemicals like 4-((Triethoxysilyl)Methyl)Morpholine leads to waste, ruined research, or even lost funding. My advice: treat every bottle as a valuable tool. Safe storage isn’t just protocol, it’s basic respect for solid science and the people doing the work.
4-((Triethoxysilyl)Methyl)Morpholine isn’t a name you hear outside of labs and production plants. This isn’t your run-of-the-mill household cleaner, either. Chemists often use it as a silane coupling agent, which means it helps different substances combine for coatings, adhesives, or advanced material manufacture. The stuff works because it has a silane end that likes to grab onto glass or metal, and the morpholine ring opens up some use in chemical engineering. But the same features that make it useful also mean it deserves genuine respect in the workplace.
This chemical brings with it a bag of hazards. Triethoxysilyl groups hydrolyze quickly in the presence of water, splitting into ethanol and producing silanols. Ethanol vapor isn’t just flammable—it can sneak up on workers unprepared for its presence, especially in closed lab rooms or storage spaces. Speaking from experience, it only takes a single rush job, forgetting to check air flow or wearing the wrong gloves, to learn that even small leaks matter.
Morpholine rings, on the other hand, come with their own set of risks. Many morpholine derivatives cause skin and eye irritation. If a drop ends up on your skin or you get a whiff of its harsh vapor, irritation and sometimes allergic responses show up fast. The chemical can also cause respiratory issues if inhaled as a mist or vapor, a risk in any place where significant quantities get poured, mixed, or transferred. Inhalation can happen more easily than folks realize, especially since morpholine doesn’t always smell strong until concentrations rise.
Safe handling calls for a culture of respect for the material and an understanding of chemical basics. Rubber gloves offer good defense, but nitrile gives stronger protection for morpholine derivatives. Splash-proof goggles and a full face shield beat out basic safety glasses. Breathable air isn’t just a nice-to-have; a working fume hood belongs in every room used for work involving this substance. Getting too comfortable—treating the chemical as just another bottle on the shelf—courts trouble, because vapors and droplets travel farther than most eyes can catch.
Storage also demands attention. Containers keep best in tightly sealed, original packaging. Once those silane groups meet moisture, the resulting ethanol can increase vapor pressure inside bottles. Pressure buildup can deform caps or start slow leaks that nobody notices until a strange smell starts to spread. Keep it far from water sources and acids to avoid runaway reactions. Non-sparking tools and explosion-proof refrigeration help prevent any accidental fire, which could turn minor mishandling into a larger emergency.
A few checklists don’t replace real safety training. The best teams treat chemical handling as an everyday skill, not a boring chore. New lab workers benefit from demonstrations before they ever touch the product. Supervisors review safety data sheets each batch, looking for manufacturer updates. Eyewash stations and spill kits get checked, not just installed.
If spills or minor contamination happen, washing with soap and water right away reduces harm. Medical attention should be immediate if symptoms develop after exposure, especially for the eyes or airways. The real risk comes from cutting corners, especially when moving quickly between projects or in high-pressure environments.
Better awareness can save health and money. Lab managers might consider vapor sensors and real-time air monitors to catch ethanol or morpholine fumes. Regular, honest reviews of incidents—even near-misses—push a culture that values prevention. Manufacturers keep improving packaging to minimize spills and slow leaks, which helps protect everyone from warehouse to final user.
4-((Triethoxysilyl)Methyl)Morpholine delivers value in industry but expects hands-on care. Every bottle is a reminder—safety belongs at the bench, not out in the hallway.
4-((Triethoxysilyl)Methyl)Morpholine lands on the list of specialty silanes that industries rely on for surface modification. From my own time handling chemical inventories, nothing throws a wrench into a workflow like discovering a bottle past its prime. Everything from epoxy coatings to adhesion promoters can see setbacks if the building blocks show signs of age. This chemical, with its complex mix of silane and morpholine, brings both power and fragility to storage cabinets.
Drawing from manufacturer specifications, most unopened containers of this silane last between twelve and twenty-four months. Those numbers take shape in the real world only with proper storage—sealed containers, cool rooms, low humidity. Even in the most high-tech warehouses, silanes tend to start breaking down as soon as moisture sneaks in. Opening the cap, just like leaving milk out, invites trouble. Ethoxy groups in the molecule react with air and water. I’ve seen batches gel up or lose performance well before the sticker date when labs forget basic precautions.
Letting a bottle sit for too long invites hydrolysis and polymerization. That means a silane that was once crystal clear could suddenly form a haze or settle into solids at the bottom. From my time troubleshooting adhesion failures, expired silane usually tops the list of suspects. Those silanol groups formed through slow hydrolysis actually mess with the effectiveness, as they don’t bind to surfaces the way you want.
In practice, temperature swings often cut shelf life in half. Even a storeroom that creeps above 30°C shortens the usable period to less than a year. Leaving a cap loose during project rush means the next technician gets a gummed-up mess. Dry, cool, and airtight conditions matter more than any label date. Personally, I always push for silica gel packs in storage cabinets for silanes. It’s a small step that can pay off, especially with specialty chemicals that fetch a premium.
Routine checks and labeling make the difference between an efficient lab and a hazardous one. Relabeling every container with both receipt and opened dates helps keep track. Regular visual inspections—looking for clarity, sediment, or color change—catch problems before anyone uses outdated stock. Training staff to recognize changes in viscosity or odor teaches vigilance. Too often, people think shelf life is someone else’s concern.
Suppliers with good credibility offer technical sheets and support lines for questions about expiry. Few things beat calling a chemist for advice rather than guessing from a faded label. Choosing smaller container sizes for slow-moving inventory also keeps wastage down.
Ignoring expiration on chemicals such as 4-((Triethoxysilyl)Methyl)Morpholine opens the door to costly product failures, unpredictable reactions, and safety incidents. I’ve watched projects come grinding to a halt over a couple grams of expired silane. The lesson? Shelf life deserves respect, not just as a number but as a key factor in efficiency and safety. By understanding the limits and responding to them, teams keep quality high and risks low, which makes every day in the lab a little smoother.
I’ve spent years watching people underestimate what can go wrong with specialty chemicals. 4-((Triethoxysilyl)Methyl)Morpholine likes to show its full personality during mixing and storage, but it rarely comes with a warning label about its quirks. This compound combines the silane structure—prone to hydrolysis—with morpholine, a nitrogen heterocycle seen in everything from surfactants to corrosion inhibitors. Put together, this molecule offers unique benefits but could introduce more risk if handled carelessly.
Triethoxysilane groups never keep secrets. They break apart quickly in the presence of water and even in humid air, producing ethanol and reactive silanol species. This may sound harmless, but anyone who’s seen a gummed-up mixing vessel after an unexpected hydrolysis event knows better. Keeping dry conditions isn’t just a suggestion; it’s the only way to keep storage and process equipment clean and functional. Glassware develops a foggy film fast, and downstream products lose consistency or even fail, so controlling atmospheric exposure becomes the first real defense.
Adding acids or bases to solutions containing 4-((Triethoxysilyl)Methyl)Morpholine speeds up the hydrolysis and condensation reactions. Acidic conditions crack open the silanes, while strong bases push the morpholine ring or silyl ether linkages toward side reactions. Unexpected polymerization or gel formation ruins entire batches in coatings labs, and pipes can clog before anyone has a chance to flush them. Some chemists try to buffer reactions, hoping for a gentle nudge rather than a full-scale runaway reaction, but if you’ve ever cleaned out epoxy lines, you know accidents rarely stay contained. Polymers that look perfect in a beaker start separating or crosslinking at scale with the wrong catalyst. Staying mindful of this mixed chemistry—recognizing both the organosilane and morpholine as reactive hot spots—makes a difference.
Certain metals make poor partners during storage and synthesis. Aluminum, zinc, and tin corrode quickly when trace moisture triggers the release of ethanol and silanol. Over time, that eats away seals and joint connections. Switching to glass, high-grade stainless steel, or proper plastic liners means one less headache for maintenance teams. I’ve seen ordinary steel tanks pitted and ruined even when folks swore they kept them dry. It doesn’t take much moisture to start the process.
Putting several functional silanes together feels efficient but introduces more room for error. Each one responds differently to water, heat, and pH. Some crosslink too early, leaving uneven product or clogging filters. The morpholine group can interact with other amines or acids in the blend, sometimes causing layer separation or unexpected cloudiness. Batch records and pilot runs help, but the real trick involves walking the shop floor—watching mixtures for hints of viscosity change or haze, and sampling more often than the textbook says. Those small signs point to looming incompatibilities no spec sheet ever fully covers.
A Material Safety Data Sheet (MSDS) covers basic hazards, but the practical side lives in daily habits. Running dehumidifiers, double-checking joints for leaks, purging vessels with nitrogen after cleaning—these steps keep the quirks of 4-((Triethoxysilyl)Methyl)Morpholine from turning into production halts. Involving technical staff in routine checks uncovers those slow burns in reactivity before they threaten timelines.
Better results come from building a workflow that treats every specialty chemical as a living thing, prone to shifting moods depending on the mixture and conditions. Daily walkarounds, rigorous notes on small deviations, and quick communication across teams give everyone a chance to prevent chemical reactivity from turning into profit-draining scrap.
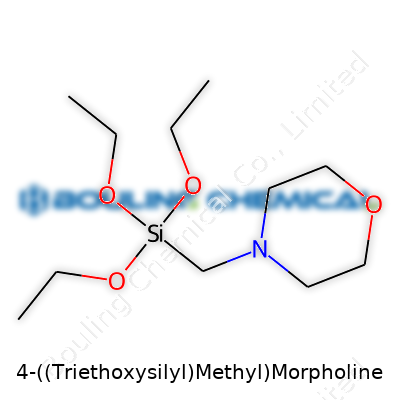
| Names | |
| Preferred IUPAC name | N-[[(Triethoxysilyl)methyl]morpholine |
| Other names |
4-(Triethoxysilyl)methyl)morpholine Morpholine, 4-[(triethoxysilyl)methyl]- Triethoxy(4-morpholinylmethyl)silane |
| Pronunciation | /ˌfɔːr ˌtraɪ.ɪˌθɒk.siˌsaɪ.li ˈmɛθ.əl ˈmɔː.fəˌliːn/ |
| Identifiers | |
| CAS Number | 124200-18-6 |
| Beilstein Reference | 1270231 |
| ChEBI | CHEBI:138552 |
| ChEMBL | CHEMBL4142675 |
| ChemSpider | 125040 |
| DrugBank | DB22024 |
| ECHA InfoCard | 18b5b4e8-6b96-408c-b211-ae3b269b3c54 |
| EC Number | 81610-76-0 |
| Gmelin Reference | 87220 |
| KEGG | C19699 |
| MeSH | D017355 |
| PubChem CID | 117830591 |
| RTECS number | RR2080000 |
| UNII | 0J9R0H14QX |
| UN number | UN3334 |
| Properties | |
| Chemical formula | C13H31NO4Si |
| Molar mass | 289.45 g/mol |
| Appearance | Colorless to pale yellow transparent liquid |
| Odor | Characteristic |
| Density | 0.994 g/mL at 25 °C(lit.) |
| Solubility in water | Soluble |
| log P | -0.2 |
| Acidity (pKa) | 7.4 |
| Basicity (pKb) | 5.98 |
| Refractive index (nD) | 1.441 |
| Viscosity | Viscosity: 7 mPa·s (25 °C) |
| Dipole moment | 3.67 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 532.7 J·mol⁻¹·K⁻¹ |
| Hazards | |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0-特 |
| Flash point | > 104 °C |
| LD50 (median dose) | LD50 (median dose): Oral, rat: >2000 mg/kg |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 200-500 |
| IDLH (Immediate danger) | NIOSH has not established an IDLH value for 4-((Triethoxysilyl)Methyl)Morpholine. |
| Related compounds | |
| Related compounds |
Triethoxysilane Morpholine 3-(Triethoxysilyl)propylamine N-(2-Aminoethyl)-3-aminopropyltrimethoxysilane 4-(Trimethoxysilyl)methylmorpholine |