The story of 4'-(Thiazol-2-Ylsulphamoyl)Acetanilide reflects both scientific creativity and a constant tug-of-war with disease control. In the early 20th century, researchers scoured chemical libraries for molecules that could serve as antimicrobial agents. The early sulfonamide drugs, such as prontosil, stunned the medical community by actually saving lives from bacterial infections, shifting the course of therapeutic approaches forever. The specific focus on thiazole derivatives emerged from the hope of enhancing the biological activity that earlier molecules had shown. By the 1960s, medicinal chemists were swapping molecular fragments, blending the sulfonamide backbone with thiazole groups, and fiddling with the acetanilide ring. The route was painstaking—yields were stubborn, side reactions plagued the process, but each improvement meant fewer limitations for patients and researchers.
4'-(Thiazol-2-Ylsulphamoyl)Acetanilide takes its place among sulfonamide-based pharmaceuticals recognized for their broad-spectrum antimicrobial power. This compound displays an intriguing balance: a thiazole ring ensuring compatibility with many biological targets, and a sulphonamide moiety responsible for the inhibition of key bacterial enzymes. Its design came from the ambition to increase bioavailability, tailoring the molecule so it survives in the body long enough to launch an effective attack against bacteria, yet minimizing negative effects on the body’s natural flora.
4'-(Thiazol-2-Ylsulphamoyl)Acetanilide usually appears as a fine, crystalline powder, with a faint yellow tint betraying the presence of the thiazole ring. Its melting point tags between 180-185°C, a narrow window that suggests high purity or consistently controlled synthesis. The molecule holds together firmly under standard storage conditions but responds to acids and strong oxidizers with notable instability. Water solubility remains low, but I have seen many protocols use co-solvents or buffers to drive it into solution for experimental or therapeutic purposes. It recombines easily with organic solvents, such as ethanol or DMSO, showing remarkable versatility when researchers want to test various delivery methods.
Quality control in the chemical industry starts with tight labeling requirements. Standard presentations include purity percentages by HPLC, moisture content, melting point documentation, and a full breakdown of spectroscopic data—mass spectra, NMR, and IR charts. Proper labeling never omits storage requirements: cool, dry, light-protected spaces extend shelf-life and reduce the risk of degradation. Labels carry warnings, batch information, detailed production dates, and traceability data. The need for accurate records plays out not only for safety, but for reproducibility in labs tasked with regulatory compliance and publication standards.
Laboratory synthesis of this molecule leans on a stepwise, deliberately assembled process. It begins with the formation of the thiazole ring from thioamide and alpha-haloketone starting points. Next, sulfonamide synthesis draws on chlorosulfonic acid, which attaches the sulphamoyl group to an aniline derivative. The acetanilide comes together by acetylation, using acetic anhydride in the presence of a mild base. Key decisions involve solvent choice—dimethylformamide and dichloromethane appear often, owing to their ability to dissolve a range of intermediates. Chemists regularly debate reaction temperature and time, aiming to maximize yield without provoking decomposition or dangerous byproducts, especially since some intermediates emit unpleasant fumes or pose fire hazards.
The molecule’s architecture is ripe for modification. I have seen groups experiment with halogenation on the thiazole ring to see how pharmacological potency might shift. Methylation on the acetamide group often increases metabolic stability. Some research teams focus on the sulphonamide nitrogen, introducing bulky substituents to boost selectivity for pathogenic over commensal bacteria. Notably, the molecule tolerates mild oxidative conditions, but harsh acids trigger ring-opening reactions that quickly degrade performance, which raises the challenge of formulation in acidic environments like the stomach.
4'-(Thiazol-2-Ylsulphamoyl)Acetanilide picks up various labels across chemical catalogs and research articles. Common synonyms include N-(4-Acetamidophenyl)-2-thiazolylsulfonamide, thiazole sulfonamide acetanilide, and several international trade names. In my own experience, researchers often resort to abbreviated codes or project tags, especially when the molecule serves as a lead compound in a series of analogues. These alternate names ease the burden of communication, but they do lead to confusion unless cross-referencing is scrupulous.
Handling this compound demands respect for personal and environmental safety. Standard protocols call for gloves, eye protection, and high-quality ventilation. Skin contact or inhalation can produce irritation, with long-term exposure possibly leading to sensitization. Disposal requirements adhere to hazardous chemical rules, especially since breakdown products pose an environmental risk. Companies implement regular training on spill response and emergency procedures. In my own lab experience, every step—right down to bench cleaning—leans on written SOPs and regular checks to comply with local, national, and international safety standards.
4'-(Thiazol-2-Ylsulphamoyl)Acetanilide enjoys steady demand in antimicrobial drug development. Pharmaceutical research departments examine it both as an active pharmaceutical ingredient and as a building block for new antibacterial hybrids. Some groups pursue its anti-inflammatory or enzyme-inhibiting capabilities, linking it to disorders beyond infection, such as autoimmune diseases or metabolic dysfunction. Veterinary science has tested its potency for livestock infections, though regulatory hurdles and residue control keep its application in food-producing animals limited. Academic labs rely on it as a scaffold for teaching structure-activity relationships in medicinal chemistry courses.
I have watched this molecule pop up in drug screening assays over the years. Teams racing to combat antibiotic resistance look for any structural advantage, whether it means a fractionally stronger grip on a bacterial enzyme or resistance to being pumped out of a microbe by efflux mechanisms. Modern R&D efforts use computational docking and high-throughput screening, trying to skip inefficient analog synthesis. Patents list claims for numerous derivatives, hoping to outpace bacteria’s own evolutionary creativity. Still, the most promising programs back up early leads with animal models and thorough toxicology screens, measuring efficacy as well as safety every step of the way.
4'-(Thiazol-2-Ylsulphamoyl)Acetanilide falls under the wide umbrella of sulfonamide toxicity concerns. Adverse reactions can run from mild allergic skin eruptions to severe anaphylaxis. Hematological side effects, such as aplastic anemia, have driven clinicians to keep rigorous records. Animal studies focus on LD50, organ-specific toxicity, and how the compound affects cytochrome P450 enzymes. Chronic exposure studies monitor onset of organ damage, reproductive effects, or carcinogenicity. Regulatory agencies demand clear data before considering approval for human or animal use. Even trace levels in lab effluent are tracked, reflecting a broad, international concern about microcontaminants.
The fight against multi-drug resistant bacteria keeps interest in new sulfonamide-thiazole hybrids alive. Machine learning models sift through molecular databases for analogs with better selectivity or reduced side effects. Green chemistry approaches could bring synthesis into alignment with modern environmental values, trimming waste and scaling up with safer reagents. Collaboration between industry, academia, and regulators will likely determine how far these derivatives go—grant funding decisions, regulatory thresholds, and market need shape the next phase. My own hope rests on the continued willingness of chemists to revisit “old” molecules for new solutions, keeping doors open for innovation, especially as global health threats continue to evolve.
4'-(Thiazol-2-Ylsulphamoyl)Acetanilide has a name few folks outside of science recognize, but it plays an important part in healthcare. Better known to most chemists under the general umbrella of sulfonamide compounds, this molecule belongs to a group of drugs once considered the backbone of bacterial infection treatment. In a world now facing superbugs and antimicrobial resistance, understanding old medicines and their uses matters just as much as chasing down new cures.
Sulfonamides aren't as fashionable today as they once were, but they set a foundation. Years ago, doctors reached for medicines like sulfathiazole – a close chemical relative of this compound – to treat pneumonia, urinary tract infections, even wounds soldiers picked up during wars. These drugs keep working by blocking the bacteria’s ability to make folic acid – something germs need to grow and multiply. That trick helped save millions before penicillin arrived.
Many folks forget: antibiotics, including these older types, helped transform hospitals into safer places. C-sections got safer. Minor cuts stopped being a life-threatening risk. In my own family, older relatives sometimes tell stories about how these “sulfa drugs” pulled a cousin or a neighbor out of what looked like terminal pneumonia during the 1940s.
Today’s medical world doesn’t often choose 4'-(Thiazol-2-Ylsulphamoyl)Acetanilide as a first-line treatment, but the story doesn’t end there. Researchers keep it in their toolkits while tackling newer, hardier strains of bacteria. Some regions with more limited access to modern antibiotics still depend on sulfonamides. Veterinary medicine also relies on these compounds for farm animal care. For some stubborn urinary infections and rare conditions, doctors may turn to a combination of these sulfa medicines.
Even outside direct patient care, understanding the chemical interactions of sulfonamides helps researchers design newer drugs. Old tools serve as reference points and learning guides, showing which parts of the molecule matter most and what to avoid because of side effects or resistance.
These medicines come with trade-offs. Allergic reactions grew pretty common in some patients. Blood disorders, kidney problems, and skin rashes popped up, especially without careful monitoring. Overuse during past decades led to some bacteria finding ways to dodge the medicine entirely. Modern doctors try to limit these drugs to the cases where benefits clearly outweigh risks.
Staying ahead of infections means not tossing out any useful knowledge. Scientists across the globe pore over old drugs, sometimes giving them new twists or combining them with modern molecules. Hospitals now use lab tests to make sure an infection will actually respond before breaking out older antibiotics. Education for patients remains crucial so that unnecessary prescriptions don’t fuel resistance.
Thiazole-containing sulfonamides like 4'-(Thiazol-2-Ylsulphamoyl)Acetanilide remind us that medical progress doesn’t always mean forgetting the past. Lessons from previous generations and careful research keep these molecules in the fight, especially in tougher situations where not much else works.
Working with tough chemicals like 4'-(Thiazol-2-Ylsulphamoyl)Acetanilide demands a clear understanding of the risks. Nobody heads into a lab thinking their day will end with a rash, a hospital visit, or a spill that lingers in the air for hours. Yet, moments of carelessness or a lack of proper preparation have tripped up even seasoned researchers. Years in the lab taught me to treat every bottle with skepticism, especially when the label included words like “dermatitis,” “sensitizer,” or “respiratory hazard.” Toxicity isn’t always visible, and delayed symptoms are real. Knowing a substance’s dangers comes down to reading the SDS sheet—boring? Sure, but it keeps the body unharmed and the conscience clear.
The hood is more than a piece of glass dividing worker from chemical. Ventilation removes vapors and airborne particles before they reach the lungs. I’ve known folks who thought a window cracked open and a room fan worked "well enough" until they learned about exposure limits the hard way. Fume hoods should always stay on. If the process stirs up fine powders or generates spray, a sash kept low and steady hands help contain the mess. Regularly testing airflow—sounds fussy—can mean the difference between safe breathing and long-term asthma.
Gloves, lab coats, and goggles aren’t for show. Nitrile gloves seal out many organics and keep skin clean, but swapping in fresh ones after each session makes sense. Wearing cotton underneath the lab coat reduces the burn if anything leaks through. Safety glasses must cover fully; even a tiny splash near the corner of the eye can cause damage. Closed-toed shoes—no sandals, ever—keep toes safe. Shared experiences show that accidents favor the unprepared.
Spills don’t just wipe up with a paper towel. Absorbent pads and neutralizing agents form the backbone of any chemical cleanup kit. Quick reporting to coworkers saves time and headaches. During one spill in a graduate lab, swift use of a spill kit and evacuation limited the impact to a few sweat-soaked minutes rather than hours of panic.
If a drop lands on skin, washing promptly at the nearest sink pushes the odds in your favor. Eyes exposed? Straight to the eyewash, using both hands to pry the eyelids open—that awkward drill sticks in memory. Once safe, medical attention isn’t optional, even for mild symptoms. Many chemicals stir up delayed reactions.
Proper storage cuts down on both risk and waste. Secure lids, clear labeling, and locked cabinets separate the harmful from the harmless. Temperature shifts and humidity cause containers to leak or degrade. Segregating acids, oxidizers, and organics spares everyone unnecessary scares. My habit of double-checking labels before pouring paid off after a labmate mixed containers by mistake—a headache avoided thanks to careful review.
Disposal deserves focus, too. Dumping into the sink rarely fits regulations. Collection in labeled containers, with documentation, prevents headaches during inspections. The environmental impact goes far beyond the building, and tracking chemical waste reaffirms respect for the world beyond the lab bench.
No short cuts. Newcomers and seasoned staff benefit from regular safety training and open conversations about near misses. Asking questions creates a work culture that values life and health. The sharpest minds I worked with all shared one thing in common—a deep commitment to their own safety and that of their colleagues, rooted in daily habits, not just policy.
Whether handling grams or milligrams, every step should run through a checklist: ventilation on, gear fitted, cleanup supplies close, label read twice. Mistakes still happen, but keeping risks front-of-mind lowers the odds of the sort of story no one wants to recount. Safety isn’t just a rulebook; it’s a way to protect both progress and peace of mind.
The name 4'-(Thiazol-2-Ylsulphamoyl)Acetanilide rolls right off the tongue only for chemists or pharmacists who came face-to-face with textbook pages filled with daunting molecular diagrams. In reality, this compound serves as a great reminder of just how intricate and creative chemical structures can get. Its presence in antimicrobial research and drug development makes it more than a chemical curiosity on paper—it represents the intersection of design and function in medicinal chemistry.
At its core, 4'-(Thiazol-2-Ylsulphamoyl)Acetanilide merges three distinct regions: a thiazole ring, a sulphamoyl group, and an acetanilide backbone. Staring at this, I remember my university days of grinding through benzene derivatives and heterocycles. The acetanilide portion carries a benzene ring with an acetyl group linked to an amide. Attach a thiazole—a five-membered ring containing both sulfur and nitrogen atoms—onto this, and you’ve got the first piece. The sulphamoyl group, a derivative of sulfonamide, bonds to both the aromatic ring and the thiazole, stitching the whole structure together at the 4' position on the phenyl ring.
Visualizing chemistry isn’t always straightforward for those who don't work with structural formulas daily. Think of this molecule as a scaffold with shapes branching off from a central frame. Each section, whether it’s the rigid aromatic ring or the more dynamic thiazole, supports the overall activity and how the molecule interacts with enzymes or bacteria. Researchers studying antibiotics or enzyme inhibitors appreciate molecules with such frameworks since each component can tweak how well the compound clings to its biological target.
Sulphamoyl derivatives paved paths for many early antibiotics. Doctors prescribed sulfonamides, relatives of this compound, to treat infections that terrified families before the 1940s. In the present, bacterial resistance forces scientists to hunt for new weapons—tweaking structures just like this one in hopes of outsmarting stubborn pathogens. The thiazole ring shows up in many drugs and earns attention for broadening how compounds interact with biological molecules.
This specific structural design helps medicinal chemists tailor molecules with increased potency or decreased toxicity. For instance, adjusting the thiazole or sulfonamide sections can affect how easily a compound dissolves, passes through biological membranes, or avoids breakdown inside the body. These factors can translate directly into how well a drug performs—whether it hits the bloodstream, whether it reaches infected tissues, and even whether patients deal with fewer side effects.
Drug resistance, environmental persistence, and manufacturing hurdles come up in any discussion of modern chemical compounds. A molecule like 4'-(Thiazol-2-Ylsulphamoyl)Acetanilide isn’t just drawn on a screen; it’s produced in labs, handled by professionals, and intended to work inside a living human or animal. Labs keep pushing to make syntheses more efficient and safer, moving away from hazardous reagents and seeking greener chemistry routes. At the same time, greater transparency around chemical exposures and downstream effects drives more rigorous safety studies, with regulators asking for robust, repeatable data.
Solving the puzzle of drug resistance won’t happen overnight. Chemists learn from compounds such as this by systematically tweaking bonds and ring structures, then sharing successful ideas across universities, companies, and regulatory agencies. Success in this area depends on strong teams—researchers, clinicians, regulators—each person bringing insight, experience, and relentless curiosity. Putting in the legwork in labs helps ensure the next generation of lifesaving drugs is smarter, safer, and more available to those who need it most.
Over the years, I’ve worked around labs where dozens of substances share shelf space. Some, like 4'-(Thiazol-2-Ylsulphamoyl)Acetanilide, ask for more respect than others. This compound, like many sulfonamide derivatives, tends to shift in purity or react if you store it in the wrong setting. Humidity or careless exposure can spoil an entire batch. Keep this compound dry. Throwing it into a cupboard with a leaky faucet above isn’t just sloppy, it actually risks changing its structure and how it performs.
Room temperature rarely means the same thing in every building. Too much heat can break bonds in even “stable” looking molecules, including this one. My best results have come from keeping these chemicals around 20°C, away from direct sunlight or the sway of outside temperatures. If a sample spends one afternoon in a sweltering storeroom, don’t expect the analysis to line up with the last time you ran it. Commercial labs often use air-conditioned storage. At home setups, even a simple insulated box and a digital thermometer make all the difference.
Some compounds fade, yellow, or break down when you leave them out in the open light. I once lost a valuable set of samples to a sunbeam that snuck past a half-closed curtain. 4'-(Thiazol-2-Ylsulphamoyl)Acetanilide falls in this group. Store it in brown glass or a well-sealed opaque container—tucked away from windows and bright lights. Even lab lighting adds up during long storage. Keeping chemicals in a dark cabinet builds a habit of caution that pays off across all substances.
Sealed containers aren’t just for liquids. Solid chemicals soak in moisture or contaminants right out of the air. I keep desiccants (those little silica gel packs) in every container holding this compound. Double-bagging inside well-fitting screw-cap jars traps even less air. This simple habit means powders stay free-flowing instead of clumping and their analysis stays accurate. Using an inert atmosphere, like a glovebox filled with nitrogen, cuts risk further for larger quantities, but for most small labs or home chemists, careful sealing often does the trick.
Anyone who’s misread a faded label knows how fast a mistake can snowball, especially with chemicals you can’t identify by sight or smell. The last thing you want is a toxic mix-up. I write out full names, dates of receipt, and any lot numbers in waterproof ink. This keeps track of age, since older samples break down faster. Don’t store this compound near acids or bases, as many sulfur-containing chemicals react badly, making the workspace safer for everyone. Separate shelves, bins, or at least divided sections inside storage areas bring down your risk.
I’ve watched too many people treat chemical leftovers like trash. Disposal rules for a compound like 4'-(Thiazol-2-Ylsulphamoyl)Acetanilide are strict for good reason—environmental safety matters. Check local regulations. Work with hazardous waste collection services rather than flushing or tossing out by accident. Care in storage runs hand in hand with proper disposal routines, completing the cycle of handling such a sensitive substance responsibly.
4'-(Thiazol-2-ylsulphamoyl)acetanilide isn’t a name you’ll hear every day at the grocery store, but this compound pops up in discussions about industrial chemicals and pharmaceuticals. Many folks probably recognize parts of the name—a thiazole ring here, a sulphonamide group there. These classes of chemicals show up in medicines and dyes, but they also sometimes bring health and environmental questions.
I’ve talked with chemists and read through safety data sheets for compounds like this over the years. The reality with something like 4'-(Thiazol-2-ylsulphamoyl)acetanilide is you always have to dig beyond the jargon. Thiazole-based compounds have been explored for their applications as drugs, antioxidants, and dyes. But even if a substance has some benefits, it’s never wise to assume it’s completely safe.
You need more than a scary-sounding chemical name to know if something harms humans or the environment. Researchers rely on studies, real-world experience, and solid data. Toxicity—meaning how much of a chemical can cause harm—depends on how it gets into the body, how much is present, and if someone gets exposed over weeks, months, or years.
Thiazole sulfonamides, the broader family that this compound falls into, served as the basis for some early antibiotics. These drugs saved lives, but they could also cause allergies. Anyone who’s ever broken out in hives from an antibiotic knows that some thiazole-containing drugs can trigger strong immune responses.
Tests with similar chemicals hint that they can cause irritation to skin and eyes, and can even affect internal organs at higher doses. Repeated exposure might damage the liver or kidneys, especially if the compound builds up inside the body. The big issue is that not every version of the compound gets the same amount of study. Toxicological profiles often stay incomplete unless regulators or manufacturers push for comprehensive research.
Manufacturers and scientists don’t always fill in every blank. Sometimes a compound goes decades with limited animal testing and minimal human exposure records. In my own experience dealing with chemical safety in university labs, the guidance always leaned cautious—assume hazard until shown otherwise. The safety data sheets I’ve handled for related sulfonamide chemicals carry warnings for respiratory irritation and organ effects, urging respect for dust and vapors.
Anyone who uses or makes chemicals like these should grab gloves, eye protection, and follow good ventilation practices. In industrial settings, there’s no good excuse to skip personal protective equipment or ignore fume hoods. Any accidental spills should be cleaned up with proper absorbents, and skin contact washed away right away. Ingestion and inhalation pose the most risk, so clear labeling and secure storage help stop accidents before they happen.
Environmental release of organic chemicals, especially those with sulfur and nitrogen, leads to questions around water contamination and breakdown products. Some related compounds don’t degrade quickly—they can stick around and build up. Regulatory agencies usually ask manufacturers for studies on biodegradability, aquatic toxicity, and long-term human health impacts before approving new chemical uses.
Better oversight and public access to toxicity studies would smooth things out. Shared databases, updated safety guidance, and open communication between chemists, industry, and regulators keep risks in check. If a compound like 4'-(Thiazol-2-ylsulphamoyl)acetanilide heads toward wider use, having that data on hand makes all the difference for safety rules in labs, factories, and the wider world.
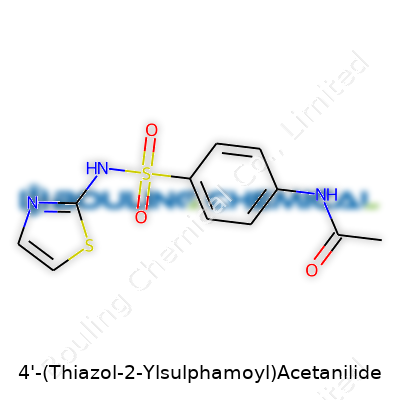
| Names | |
| Preferred IUPAC name | N-{4-[(1,3-Thiazol-2-yl)sulfamoyl]phenyl}acetamide |
| Other names |
N-(2-Thiazolylsulfonyl)-N-acetylaniline N-[(2-Thiazolyl)sulfonyl]acetanilide Thiazolsulfonylacetanilide |
| Pronunciation | /ˌθaɪəˌzɒl.tuːˈɪl.sʌlˌfæmɔɪl.æsəˈtænɪlaɪd/ |
| Identifiers | |
| CAS Number | 53949-29-8 |
| Beilstein Reference | 2949539 |
| ChEBI | CHEBI:75439 |
| ChEMBL | CHEMBL521915 |
| ChemSpider | 22517176 |
| DrugBank | DB08695 |
| ECHA InfoCard | echa-info-card-100.104.235 |
| EC Number | 3.5.4.13 |
| Gmelin Reference | C10H9N3O3S2 |
| KEGG | C07272 |
| MeSH | D000900 |
| PubChem CID | 409733 |
| RTECS number | XG8225000 |
| UNII | Y26C8S3I8U |
| UN number | 3077 |
| CompTox Dashboard (EPA) | DTXSID5020442 |
| Properties | |
| Chemical formula | C11H11N3O3S2 |
| Molar mass | 307.36 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.48 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | 0.09 |
| Vapor pressure | 4.41E-10 mmHg at 25°C |
| Acidity (pKa) | 9.9 |
| Basicity (pKb) | 8.02 |
| Magnetic susceptibility (χ) | -80.00 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.683 |
| Dipole moment | 5.74 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -308.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -995.1 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | J01EC01 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P333+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | >178°C |
| Lethal dose or concentration | LD50 oral rat 3200 mg/kg |
| LD50 (median dose) | LD50 (median dose): Mouse (oral) 1 gm/kg |
| NIOSH | RN875-74-1 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 mg/m^3 |
| Related compounds | |
| Related compounds |
Acetazolamide Methazolamide Dorzolamide Brinzolamide Ethoxzolamide |