4-Piperidylmethylamine draws its roots from the broader study of heterocyclic amines, a category of compounds that researchers started paying close attention to well over a century ago. Chemists in the early and mid-20th century realized that the piperidine ring system showed surprising versatility, and the drive to manipulate its structure opened new frontiers for pharmaceutical and chemical innovation. The specific modification of attaching a methylamine group at the 4-position was not a random step. Researchers aimed to push the boundaries of what these rings could do by tweaking their substitution patterns. Over the decades, scientists published papers showing that small changes at different positions on the ring changed the overall behavior in real and measurable ways, a trend that continues today as molecular tweaking fuels drug discovery and specialty chemical development.
In the lab, 4-Piperidylmethylamine stands out because its structure—a saturated six-membered ring with a nitrogen at the center and a methylamine branch at the 4-postion—gives it a blend of basicity and reactivity that chemists value. It falls within the toolbox of intermediates for organic synthesis. Teams studying psychoactive substances, kinase inhibitors, or even agricultural applications find it provides a bridge to more complex molecules. The commercial landscape for this compound includes vialed reference materials, bulk intermediates, and specialty reagents. This amine keeps showing up in new patent filings, thanks to its convenient modifiability and track record in synthetic pathways.
With 4-Piperidylmethylamine, you see a crystalline or oily liquid, depending upon its purity and mode of storage. Its molecular formula, C6H14N2, keeps it in the small-molecule range. It offers moderate solubility in water and mixes well with polar organic solvents, such as ethanol and dimethylformamide. The compound brings an amine-like odor, a boiling point usually falling a little above 200°C, and, as expected, shows a tendency to absorb moisture from air. As a secondary amine, it reacts strongly with acid chlorides or isocyanates, among others, and holds a basic nitrogen that will take up a proton in slightly acidic environments.
Producers shipping 4-Piperidylmethylamine usually specify a purity exceeding 98%, supported by gas chromatography-mass spectrometry or NMR analysis. Labels include the batch lot, storage instructions—often calling for a dark, cool place—and hazard rating symbols in compliance with GHS or REACH. You’ll see the molecular weight, CAS number, country of origin, and expiration date detailed. For industrial applications, certificate of analysis documents accompany each consignment so users can check for trace byproducts or residual water content. Consistency proves crucial for research and manufacturing alike. Without clear technical data and labeling, users risk running into big problems down the road if, for instance, a new lot performs differently from a previous one.
Flow chemistry and classic batch syntheses both see heavy use in making this amine. A well-trodden path starts with 4-piperidone, which undergoes reductive amination with methylamine gas or equivalent precursors. Hydrogenation—often using palladium on carbon as a catalyst—drives the reaction to completion. Skipping stringent purification steps leads to colored or impure products, so processors usually adopt vacuum distillation or chromatography to secure a defined product. Alternative routes sometimes include alkylating 4-piperidinol with formaldehyde and ammonia or methylamine in the presence of acid catalysts. This variety in preparative pathways lets labs and factories scale production up or down to suit their needs, with plenty of room for optimization to improve yield or reduce byproducts.
4-Piperidylmethylamine stretches its utility far beyond simple amination chemistry. Its reactive amine invites acylations—forming amides for peptide-like structures or linking small molecules together—and finds equal favor in formulation of carbamates or ureas. Alkylation broadens its use, and arylation provides new platforms for development of aromatic derivatives. Because both nitrogens on the molecule allow functionalization, chemists can introduce fluorescent tags or prepare crosslinked polymers that depend on multi-point N-reactivity. Once you dig into the breadth of reactions, you see pharma and agrochemical chemists leaning on its reactivity to stitch together complex scaffolds where a sturdy piperidine backbone still counts for a lot.
Producers and suppliers keep things interesting with a range of synonyms. 4-Piperidinylmethylamine pops up in registries, sometimes labeled as N-(Piperidin-4-yl)methanamine or 4-(Aminomethyl)piperidine. Technical catalogs from Europe and North America also use trade designations that fold in code numbers or supplier-specific prefixes. Regardless of naming, users recognize the importance of CAS numbers for identifying the same chemical even across international markets or procurement channels.
Handling 4-Piperidylmethylamine means following real safety protocols, something every person working in a lab or production site learns early. The compound causes moderate irritation to skin and eyes, so gloves and goggles stay standard. Inhalation can trigger respiratory discomfort—using a fume hood reduces that risk. Storage in tightly sealed containers, away from oxidizing agents and acids, keeps things stable and prevents accidental degradation. Disposal needs real attention, as local environmental policies restrict amine waste dumping. Training sessions and safety data sheets push users to review first-aid and spill management steps, further backed by periodic hazard reviews as formulations or processes evolve over time.
The reach of 4-Piperidylmethylamine cuts across drug discovery, especially in developing CNS-active compounds that need a flexible amine handle for building analogues. Crop protection chemistry uses it as a backbone in designing new insecticides or fungicides with novel action modes. Specialty materials producers experiment with it in synthesizing corrosion inhibitors or crosslinked coatings. Analytical chemists find it handy as a derivatizing agent for improving detectability of small molecules. Each field values its modular structure, with patents and publications showing that research teams leverage its reactivity to streamline workflow and cut project timelines.
Companies and academic chemists keep revisiting the piperidine core because of its adaptiveness. Ongoing work investigates new derivatives that show improved selectivity for biological targets, such as receptors and enzymes implicated in neurodegenerative disease or cancer. Some studies push the boundaries by linking multiple amines, or by attaching new side chains that improve cell permeability or metabolic stability. Research teams deploying computational chemistry model how subtle changes at the 4-position shift activity, feeding those insights back into iterative lab synthesis. The body of literature around this amine keeps growing, fueled by its low cost, ready modifiability, and record of success as a precursor in proof-of-principle medicinal chemistry.
Toxicologists scrutinize amines for a reason—for some, metabolic byproducts or reactivity toward proteins can create unwanted effects in biological systems. 4-Piperidylmethylamine itself shows moderate oral and dermal toxicity in animal studies, often cataloged in toxicology databases for reference. Symptoms from overexposure include CNS excitation or depression, as seen in other piperidine relatives. Longer-term studies look for evidence of genotoxicity, reproductive harm, or ecological persistence; results to date call for careful handling, well-marked isolation procedures, and attention to environmental release. Regulatory agencies flag the substance based on concentration in formulations, demanding clear MSDS access and responsible record-keeping by users.
4-Piperidylmethylamine finds itself in a sweet spot for future chemical innovation. The broad demand for selective CNS drugs nudges medicinal chemists to seek out new analogues built around its piperidine core, while advances in sustainable synthesis press manufacturers to develop cleaner, safer, and cheaper processes for scaling up production. Cheminformatics platforms now map structure-activity landscapes for hundreds of amine derivatives at a time, giving a data-driven edge to discovery. As researchers track green chemistry trends, they look at biocatalysis and continuous flow as promising directions for reducing hazardous waste and improving atom economy in preparing this molecule. The collective drive to push boundaries in neuroscience, materials, and even catalysis ensures that this amine and its derivatives remain priority candidates for research and commercial application alike.
Talk to any lab worker who juggles chemical catalogs and you’ll hear about 4-Piperidylmethylamine. It’s a mouthful, but this compound actually plays a practical role in scientific research and industry. 4-Piperidylmethylamine has a molecular structure that attracts the attention of chemists for a reason. The piperidine ring and the methylamine group blend in a way that lets this compound serve as a key building block. If you’ve ever gone through the process of synthesizing fine chemicals or pharmaceuticals, you might have seen this name on your ingredient list.
Pharmaceutical research doesn’t move forward without reliable building blocks. 4-Piperidylmethylamine is one of those hidden, behind-the-scenes ingredients that supports medicinal chemistry teams. Chemists use it to create molecules that show promise as new drugs, especially in early-stage discovery. Its nitrogen-rich structure allows it to combine with other molecules, which helps labs produce compounds targeting neurological conditions and other disorders.
Big pharmaceutical companies often screen thousands of chemical fragments to find drug candidates. The versatility of 4-Piperidylmethylamine makes it valuable in creating those libraries. You can picture it as a Lego piece with special connectors. Put it with other building blocks, and suddenly you have a brand-new molecule—one that could fit the lock of a disease-related enzyme. I’ve worked with research teams who rely on flexible and reliable precursors, and those who run out of something as fundamental as this can face days or even weeks of delays.
Research labs and industrial chemists lean on 4-Piperidylmethylamine for more than drug work. This compound works as a starting point in the synthesis of chemicals used in agriculture, coatings, and dyes. Custom synthesis companies build out more advanced chemicals using reliable precursors like this one. Its nitrogen atoms mean reactions proceed under relatively mild lab conditions, helping scientists avoid the harsher reagents that can be tough to handle or dispose of safely.
In chemical manufacturing, process efficiency saves time and money. Factories working with piperidine derivatives count on access to compounds that react predictably. I’ve spoken to chemical engineers who design continuous flow reactors, and their jobs rely on small molecules like 4-Piperidylmethylamine being available in bulk and in the right purity. Quality control teams check these ingredients carefully, since impurities can turn a successful reaction into a batch of waste.
4-Piperidylmethylamine, like many amine-class chemicals, requires thoughtful handling. Its vapor and liquid can irritate skin or mucous membranes. Labs handling it follow material safety guidelines, using gloves and fume hoods. Some countries regulate sales or distribution of chemical intermediates to prevent misuse, so suppliers keep paperwork tidy. Chemical stewardship depends on everyone respecting safe practices, a lesson learned by many after even small lab accidents.
Research communities stress ethical use of chemicals throughout the supply chain. Reliable sourcing and full documentation support not only legal compliance but public trust in scientific progress. The days of unmarked bottles and guesswork are over. Responsible handling starts long before a molecule appears in a laboratory notebook and continues into its final application, whether in medicine or industry.
Chemistry often feels like a maze. Every corner introduces a new molecule that packs its own recipe of atoms, bonds, and ring systems. 4-Piperidylmethylamine stands out as one of those compounds that’s easy enough to draw, but still rarely lands in everyday conversation. Its structure centers on a six-membered ring, or piperidine, where nitrogen takes the place of a single carbon, changing the whole behavior of the system. On the fourth carbon of this ring, a methylamine group attaches itself: that’s a single carbon chain ending in an amine, which just means a nitrogen with a couple of hydrogens hanging off.
Let’s break it down without dragging through textbook vocabulary. You start with piperidine, a ring-shaped structure familiar to many drug chemists. It’s like cyclohexane, but stuff in a nitrogen atom for one of the ring’s carbons. At spot number four on this ring, count the atoms and land on the right place, there’s a side branch: a carbon, holding tightly to a nitrogen with two hydrogens (–CH2NH2). In chemical shorthand, people call this C6H14N2. Its systematic name might feel long, but every word means something useful. “1,2,5,6-tetrahydropyridine-4-methylamine” hardly rolls off the tongue, but it helps chemists stay precise.
From the standpoint of a curious scientist, structure isn’t just about lines on a page. Little tweaks—in this case, putting an amine on the ring—open up opportunities. Medicinal chemists love building on backbones like piperidine. That simple six-membered nitrogen ring is found all over blood pressure medicines, antipsychotics, and antivirals. The methylamine extension turns the molecule into a starting point for making other, more complicated compounds. Sometimes this branch offers new ways to latch onto receptor targets in the body or create fresh interactions that would never happen with the basic ring alone.
Labs keep tight control over piperidine derivatives. Simple changes in chemical structure can pull a substance in directions that help medicine—or, unfortunately, illicit drug synthesis. The methylamine group in 4-piperidylmethylamine can serve as a handy anchor for chemists, but it also means keeping an eye on legality and safety. Its presence means more reactivity in synthesis, which calls for safe handling and proper training. The more time I’ve spent in the lab, the more I’ve seen how one small change on a ring can cause big effects down the line. Chemicals like this don’t belong in untrained hands.
Modern chemistry means bringing together both advances in science and awareness of responsibility. 4-Piperidylmethylamine shows how a small change—like one methylamine hanging off a ring—can spark big ideas in pharmaceutical research. The key lies in combining the technical know-how of synthetic chemists, the oversight of regulatory teams, and a clear plan for safety. That approach delivers progress everyone can trust.
Some chemicals grab attention for their potential uses in everything from medicine to industry. 4-Piperidylmethylamine fits into this group, as it serves as a building block in the synthesis of pharmaceuticals and research chemicals. Curiosity about buying it usually bubbles up when people hear about its roles in labs or the headlines mention drug regulation.
You don’t stroll into a pharmacy or surf a mainstream e-commerce site and leave with a bottle of 4-Piperidylmethylamine in your cart. Its availability is woven tightly with chemical controls. The reason lies in its connection to the development of certain psychoactive substances. The authorities—such as the Drug Enforcement Administration in the United States or their equivalents elsewhere—watch the movement of chemicals like this with a close eye. More steps and paperwork follow if someone wants to buy even a small amount.
I worked for a few years in a university chemistry lab, where our inventory included a variety of amines for research. Getting our hands on them was never a snap. We submitted written justifications, safety precautions, and permits. Only certified personnel could handle storage and disposal. It was never about making things hard on purpose. The controls exist because stories of chemical misuse are real. In 2022, for example, a report from the European Monitoring Centre for Drugs and Drug Addiction described how similar intermediates found their way into illegal manufacturing. One careless sale on the open market could fuel a chain of illegal labs and harm countless people.
Suppose 4-Piperidylmethylamine ended up in the wrong hands—news of street labs using it to cook up novel designer drugs wouldn’t take long to surface. In tight-knit research circles, the vibe always leaned toward safeguarding everyone (ourselves included) from accidental contact or criminal misuse. Something as small as failing to lock a storage cabinet led to disciplinary action and, in some cases, police notification. It’s about public safety, not red tape.
Online, many chemical suppliers list 4-Piperidylmethylamine among their products. Before striking up any deal, reputable suppliers demand documentation, proof of institutional affiliation, and intended scientific use. Fly-by-night operations sometimes pop up on the dark web or unregulated overseas markets, sidestepping the rules. Buyers thinking of cutting corners risk criminal charges, health hazards, and ripped-off wallets.
Scientists and industry professionals need access to compounds like 4-Piperidylmethylamine to keep research and medical innovation moving. Regulators could push for stronger digital tracking, cross-border cooperation, and public databases of legitimate suppliers to make things safer and more efficient. Universities and companies can expand training, so new researchers understand legal boundaries and consequences.
Transparency from both regulators and suppliers helps people spot risky situations and avoid accidentally breaking the law. I recall a time a friend tried to order a common solvent for a home experiment, not realizing it sat on a regulated list. He ended up on a government watchlist for months, a lesson in reading the rules closely.
Most people searching for 4-Piperidylmethylamine aren’t trying to skirt the law—many just want solid information. Google’s guidelines highlight the importance of trustworthy, experienced voices. Health and science stories demand context, facts, and a willingness to lay out the risks as they are, not how we wish them to be.
If someone genuinely needs access to 4-Piperidylmethylamine, the right path runs through official channels and verified suppliers. Critically, education and transparency offer the best shot at keeping science open while blocking chemical misuse at its root.
4-Piperidylmethylamine isn’t just another laboratory compound. This chemical brings real hazards to the table, especially for folks used to working at the bench. In my years across academic and industry labs, I’ve seen mishandling even from veteran technicians who let routine dull their sense of caution. Contact with skin can cause irritation, and the vapors sting eyes and nose. Without precautions, exposure risks pile up fast.
Wearing gloves and safety glasses is not a mere suggestion; plenty of reports show that 4-Piperidylmethylamine absorbs through skin and causes burns. I once watched a colleague brush off “just a little splash” and regret it after a few minutes—red, sore skin taught the lesson better than any safety sign. Lab coats should fit well, never loose cuffs or hoods that can catch on glassware. Open-toed shoes invite disaster if anything spills, especially with this chemical, so only sturdy, closed footwear makes sense.
Working in a fume hood blocks the vapors that drift so easily from open containers. You want clear airflow and a working sash. I trust my nose—I can smell trouble before numbers show up on air monitors. Ceiling ventilation does not cut it; only localized extraction ensures those fumes disappear before they hit your lungs. Shortcuts here mean headaches and worse.
I keep only what I need on the bench. Spills happen less when containers stay sealed and organized. Labels in bold marker, noting hazard info, make it easier for anyone—especially newcomers—to react fast if something goes wrong. I keep spill kits at arm’s reach, not just under a forgotten sink. If a bottle tips or breaks, there’s absorbent material, gloves, and neutralizer powder handy. Hesitation in a crisis often comes from not knowing where things are.
In every lab I’ve run, I insist that no one touches 4-Piperidylmethylamine without a walkthrough. Talking about safety during onboarding is not enough. People learn by doing, shoulder to shoulder, so I show how to test fit of gloves, adjust the hood sash, and dispose wipes or glass in proper bins. Every six months, I run a drill and review what to do if exposure happens: eyewash stations and showers go unused for months, and when panic hits, muscle memory beats faded posters.
Chemical storage can slip down the priority list. I’ve seen corrosives tucked away next to snacks (not kidding). 4-Piperidylmethylamine belongs in locked, ventilated cabinets, away from acids and oxidizers. Containers get inspected for cracks or leaks at least monthly. Coding with color or big hazard symbols helps folks who can’t read the small label text when things get tense.
Dumping down the drain is never an option. Local waste regulations spell out what’s allowed. In every lab I’ve managed, a trained waste officer oversees the disposal bin, logging every transfer so nothing slips through the cracks. Mixing incompatible waste led to a fuming mess in a university lab once—no one wants that repeat.
People who understand molecules sometimes forget common sense matters most. Safety only works if everybody keeps up their end, shares what works, and owns their mistakes. Mistakes hurt less when the system supports learning instead of just blame. Respect for the risks of 4-Piperidylmethylamine grows with every shift; only diligence lets us go home in good health.
Every working chemist spends plenty of time staring at numbers, but those numbers aren’t just trivia—especially when a compound like 4-Piperidylmethylamine lands on your desk. Its molecular weight, 128.21 g/mol, acts as a key that unlocks straightforward decisions about scale, safety, and research outcomes. Tossing that number into a reaction lets you figure out how much of everything to add without flying blind. During my time in the lab, getting this step right made the difference between clean results and a worthless batch.
4-Piperidylmethylamine may not be famous outside organic chemistry circles, but structure tells a good story. It’s got a piperidine ring—basically a six-member chain with one nitrogen—plus a methylamine arm. This structure brings certain reactivity and potential uses as a building block for drugs or specialty materials. The molecular weight isn’t just a trivia answer—it gives pharmaceutical researchers and industrial chemists a hard fact they rely on for everything from purity calculations to drug formulation.
I remember running through calculations for a preclinical synthesis. Accuracy wasn’t optional. Weight out too much or too little of your base ingredient, and the whole project goes sideways. Finding the correct molecular weight is more than punching numbers on a calculator. You check, double-check, tally hydration or salt forms, and make sure the structure matches the registry. Errors cost time, money, and more than a few headaches.
Some might shrug off a point or two on the molecular weight, believing it’s close enough. I’ve seen research budgets bled dry because someone miscalculated a starting mass. This small error meant the solution’s concentration was off, setting off a chain reaction of faults in testing and quality control. It’s tough to regain trust with regulatory agencies or clients if you let simple math slip through the cracks. The number 128.21 is more than just digits; it’s tied to people’s livelihoods and company reputations.
Digital databases such as PubChem or ChemSpider lay out molecular weights, often with related data and structural info. These tools don’t just help you confirm the precise mass; they encourage transparency and reproducibility. Open access data that’s continuously vetted by experts makes the work faster and less prone to errors. Having trusted sources matters, especially when patent applications, grants, or audits ride on every calculation.
Manual checking still matters too. Anyone involved in chemistry—researchers, industrial operators, even advanced students—should cross-reference with certificates of analysis and supplier data, not scraping details from just any site or old textbook. Regulations keep evolving, and chemicals often come as salts, hydrates, or mixed batches. That extra layer of diligence turns a simple number into a guarantee.
Tuning into details like molecular weight could save months and serious money. Training lab teams to value accuracy at every step creates a culture of quality and pride in the work. With 4-Piperidylmethylamine and countless other compounds, starting from the right numbers keeps research ethical, efficient, and trusted. Getting people on board with these habits shapes not just outcomes but entire careers. I’ve seen plenty of projects succeed or fail on groundwork this simple, and it always circles back to respecting the details.
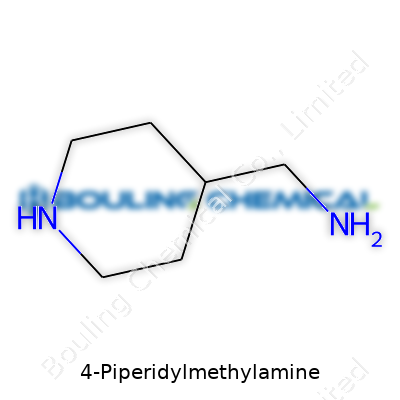
| Names | |
| Preferred IUPAC name | N-(piperidin-4-ylmethyl)amine |
| Other names |
4-(Aminomethyl)piperidine Piperidine-4-methanamine 4-PMA Piperidin-4-ylmethylamine |
| Pronunciation | /ˌfɔːr paɪˈpɛrɪdɪlˌmɛθəlˈæmiːn/ |
| Identifiers | |
| CAS Number | 4395-98-6 |
| Beilstein Reference | 1721810 |
| ChEBI | CHEBI:84954 |
| ChEMBL | CHEMBL147831 |
| ChemSpider | 126598 |
| DrugBank | DB08798 |
| ECHA InfoCard | 100.123.899 |
| EC Number | 200-419-2 |
| Gmelin Reference | 109900 |
| KEGG | C06296 |
| MeSH | D010930 |
| PubChem CID | 114119 |
| RTECS number | TK8580000 |
| UNII | DI8K3F964U |
| UN number | UN2735 |
| CompTox Dashboard (EPA) | DTXSID0014316 |
| Properties | |
| Chemical formula | C6H14N2 |
| Molar mass | 128.21 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Amine-like |
| Density | 0.954 g/mL |
| Solubility in water | Soluble in water |
| log P | 1.14 |
| Vapor pressure | 0.19 mmHg (25°C) |
| Acidity (pKa) | 10.8 |
| Basicity (pKb) | 3.38 |
| Magnetic susceptibility (χ) | -64.7×10^-6 cm³/mol |
| Refractive index (nD) | 1.496 |
| Dipole moment | 2.83 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 237.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -23.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4186 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS06,GHS08 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes severe skin burns and eye damage. Causes serious eye damage. Harmful if inhaled. |
| Precautionary statements | P264; P270; P271; P301+P312; P330; P405; P501 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 100°C |
| Autoignition temperature | 330 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 570 mg/kg |
| LD50 (median dose) | LD50 (median dose): 325 mg/kg (rat, oral) |
| NIOSH | TE1750000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 30 grams |
| Related compounds | |
| Related compounds |
Piperidine 4-Piperidone 4-Piperidylacetic acid N-Methyl-4-piperidylamine 4-Piperidinemethanol |