Back in the early 1900s, German chemists began exploring nitrogen-containing ring systems, and 4-Piperidinol soon caught their attention. Its structure—piperidine with a hydroxyl attached—offered new chemical opportunities beyond simple ring amines. Over time, labs moved from pencil-and-paper reaction notes to clockwork synthesis using more reliable equipment. 4-Piperidinol’s flexible core structure found a home both in academic labs and industry benches. By the late 20th century, the pharmaceutical focus on heterocycles pushed this compound straight into the limelight, as researchers found that one tweak on the ring altered biological activity in profound ways.
4-Piperidinol doesn’t stand out in a bottle—it’s a pale, almost colorless liquid or solid at room temperature. Most commercial supplies ship as crystalline solids, sometimes in small glass vials with labels from chemical suppliers. I’ve pulled a bottle from a storeroom shelf, and the distinct musty odor hits immediately—no mistaking it for anything else. Chemists working in drug discovery or agrochemicals keep it handy for late-stage functionalization or scaffold modifications. Its reach goes past synthetic routes—it finds a role as a chemical intermediate, helping researchers stitch together more complex molecules.
4-Piperidinol brings a molecular formula of C5H11NO to the table. A melting point sits near 37–40°C, and it boils at 215°C under normal pressure. It dissolves well in water and polar solvents, thanks to both the nitrogen and the alcohol group. The pKa of the alcohol falls near 14, while the ring nitrogen stands slightly less basic than a typical amine. Handling requires gloves—not only for safety but to avoid skin contact, as it seeps through latex easily. The hydroxyl sticks out, a ready site for further chemical tricks, and the piperidine ring holds up under moderate acidic or basic jobs.
Bottles list the compound as 4-Hydroxypiperidine or 4-Piperidinol, usually alongside a CAS number—96-56-0. Purity climbs past 98% in most research-grade samples. Labels note storage below 25°C, away from moisture. Chemical catalogs provide hazard pictograms—corrosive, acutely toxic. The safety data sheet warns about eye, skin, and respiratory irritancy, all relevant for anyone spending days rotating flasks or transferring drops. Analytical labs use NMR, GC-MS, and IR to confirm batch quality, often demanding a single dominant peak in chromatograms.
Lab-scale prep often starts with piperidone, setting the stage for reduction. Catalytic hydrogenation brings down the carbonyl, yielding 4-Piperidinol. I’ve run this route on a bench—pressure reactor, palladium on carbon, hydrogen flow, a game of patience. Some methods avoid hydrogen gas, using sodium borohydride for gentler reduction at the expense of scale. Industrial players push different routes: either via hydrolysis of 4-cyanopiperidine or via amination of 1,4-butanediol with ammonia in the presence of catalysts. These bulk processes feed chemical manufactories that can push out kilograms for further synthesis work.
The hydroxyl group offers a solid point of departure—esterification, ether formation, or oxidation to a ketone. Substitution at the nitrogen (N-alkylation) tailors the biological profile, vital in pharmaceutical development. 4-Piperidinol can swing through Mitsunobu reaction or tosylation to make better leaving groups. More adventurous chemists leverage the ring for cross-coupling, forming bonds to aromatic systems or extending to spirocyclic derivatives. The chemical toolkit for this scaffold keeps expanding, and students cutting their teeth in synthetic labs learn its flexibility early. Epoxidation of the ring, reduction, acylation—the routes continue to multiply every year.
Chemical catalogs and research articles toss out different names. Along with 4-Piperidinol, you run into 4-Hydroxypiperidine, piperidin-4-ol, and sometimes PIP-OH. The CAS registry—96-56-0—anchored in reaction databases, ensures everyone talks about the same molecule, even across languages. Commercial products may list these on secondary labels, helping avoid costly mix-ups on busy workbenches.
Work with 4-Piperidinol always calls for safety goggles, gloves, and efficient fume hoods. Skin and eye splashes sting and can cause ongoing irritation. Inhalation generates headaches or nausea; longer exposures risk more severe effects. Bigger labs invest in vapor monitors and training modules to keep technicians smart and safe. Waste handling follows local chemical disposal codes—neutralization in controlled containers, no casual rinsing down the drain. A single oversight clogs up entire workflows, and everyone quickly learns respect for safety data.
The biggest impact hits medicinal chemistry and fine chemical production. Medicinal chemists draw on 4-Piperidinol to build antihistamines, antipsychotic drugs, and enzyme inhibitors. Agrochemical researchers transform it into bioactive compounds that regulate pests or weeds, supporting sustainable crop yields. Beyond life sciences, it shows value in polymer research, acting as a flexible monomer for specialty materials. I’ve seen production teams adopt it for textile auxiliaries, using the polar hydroxyl to shape fiber properties or aid dyeing. Each new application unlocks different challenges—patent strategy, analytical purity, market regulation.
Academic labs use 4-Piperidinol in SAR (structure–activity relationship) studies, pinning down lead compounds for disease treatment. The piperidine ring remains a staple in CNS pharmaceutical research. Teams at biotech firms push analogs of 4-Piperidinol through early animal testing, looking for safer therapies or better delivery. Material scientists try out functionalization strategies—polymers, coatings, resins—all powered by the adaptability of the basic structure. Instrumental advances such as automated flow reactors let chemists process new analogs in high-throughput mode, testing more ideas per week than was possible just a decade ago.
Scientists don’t take 4-Piperidinol’s safety for granted. Acute toxicity studies in mice and rats help to establish dose-dependent profiles. Oral exposure causes irritation, CNS depression, and can lead to respiratory issues at higher doses. The compound’s metabolism routes break it down into less harmful byproducts in mammals, but the potential for chronic toxicity stays under investigation. Some cell studies report moderate cytotoxicity, setting it apart from more benign ring alcohols. Long-term exposure studies aim to pin down risks for those involved in industrial scale production. Each year, refinements in analytical monitoring reduce the possibility of unnoticed exposure, but vigilance never loses value.
The future for 4-Piperidinol depends on new frontiers in medicinal chemistry and advanced materials. Several drug pipelines now explore piperidine scaffolds to modulate protein targets unreachable by older compounds. Combinatorial synthesis continues to diversify the possible derivatives, and AI-driven molecular design tips the odds toward smarter, safer analogs. Sustainable chemistry trends push companies to explore greener production—catalyst recycling, low-energy routes, and bio-based feedstocks. A compound with a century-old pedigree adapts and thrives, finding new relevance each year in both the lab and in industrial settings. Teams that once glanced over 4-Piperidinol for flashier molecules now turn back, recognizing in its structure a set of possibilities still far from exhausted.
4-Piperidinol doesn’t grab headlines, but chemists know it well. The clear liquid’s main claim to fame comes from its role as a building block. In short, it helps people make bigger, more complicated chemicals. I’ve seen it show up in labs, usually tucked away on a shelf with other modest but mighty molecules.
If you have to point to a single reason why 4-Piperidinol matters, it starts with medicine. Many drugs need a strong skeleton before doctors even dream of the benefits. Chemists rely on 4-Piperidinol for exactly that. It supplies the basic shape for drugs that fight disease.
This compound serves as the starting point for antihistamines and certain antipsychotics. Take paroxetine, a popular antidepressant—its chemical story starts with piperidine chemistry. That means people dealing with mental health symptoms might trace some relief back to chemicals like 4-Piperidinol.
To most people, pills and creams look straightforward. But inside pharmaceutical factories, teams tinker with molecules to optimize safety and strength. 4-Piperidinol walks in early. It holds the right balance of reactivity. Chemists tweak certain parts of this compound, making it bend to their needs. By the end, the original structure works like sturdy scaffolding for whatever treatment they’re shaping.
This versatility makes 4-Piperidinol valuable, but not everything ends up as a finished drug. Research labs often explore fresh ideas by fusing pieces of 4-Piperidinol with other substances, hoping to spark something new. For every winner, plenty of failed experiments pile up along the way. Scientific progress runs on this quiet trial-and-error—4-Piperidinol just happens to be a frequent starting point.
You might not spot 4-Piperidinol at home, but some of what you use starts with it. Agrochemical companies use it to craft pesticides and herbicides. I’ve heard of its use in making strong additives for rubber and certain plastics. It doesn’t lead on any label. Instead, it shapes ingredients behind closed doors in industrial plants.
If you’ve ever used a disinfectant or a specialty cleaner at your job, there’s a chance you handled something crafted from the same roots.
Knowing that 4-Piperidinol plays such a crucial role, you’d expect oversight. Of course, the chemical comes with safety risks. Contact with skin or breathing it in can irritate, so handling requires simple protective gear. The risk isn’t news to trained workers, though—what complicates things is illicit use. Some illegal drug labs use compounds with similar structures for more harmful products. That’s led to tighter sales controls in some countries, which slows down legitimate research and industry projects.
Companies working with 4-Piperidinol now put more energy into tracking its distribution, storing it safely, and following regulations to keep the good projects moving. I think industry would benefit from clear, practical guidelines. That would let researchers carve new paths without tripping over unnecessary barriers, while still stopping folks who’d use the compound for harm.
With all its uses lined up, 4-Piperidinol never takes center stage, but it never leaves the wings either. Industry and research groups now share the task of balancing safety, access, and invention. It’s not a glamorous job, but it makes modern chemistry possible.
Ask any chemistry student about 4-Piperidinol and you’ll probably get a brief pause before the answer. It belongs to a group of molecules called piperidines. Picture a six-membered ring made of five carbon atoms and a nitrogen—that’s piperidine, a structure you’ll run into often in pharmaceutical conversations. Attach a hydroxyl group (-OH) to the fourth position on that ring and you get 4-Piperidinol, also known as 4-hydroxypiperidine. The full chemical formula reads C5H11NO, and the structure looks a little like a bicycle wheel with a handlebar.
My interest took off one late night in a friend’s organic chemistry lab, where small structural tweaks held the secret for entire rows of medicines. 4-Piperidinol kind of sneaks up in these research projects; people reach for it to build more complicated pharmaceuticals, especially antipsychotics and painkillers. The nitrogen in the ring and the hydroxyl group offer chemists hooks for adding new pieces, shaping molecules that target brain chemicals. Real people, battling everything from schizophrenia to chronic pain, often feel the difference, even if they’ve never heard its name.
Let me try to paint the picture. You have a hexagonal ring with a nitrogen atom occupying one of the spots—think of it as a clock face with nitrogen at 12 o’clock. The other five points are carbons. The number four carbon, if you count clockwise, carries the hydroxyl group. This subtle difference—a single oxygen atom and two hydrogens—totally changes the way the ring behaves, especially in the presence of other chemical partners. Chemical handbooks and 3D modeling apps show the same layout: a puckered ring with that unmistakable –OH stick out like a thumb.
The everyday person probably doesn’t bump into 4-Piperidinol unless they tune into the science behind medications. Yet its chemical structure—simple, sturdy, with some flexibility—lets drug makers move in remarkable directions. All it takes is one clever tweak to that ring, and suddenly a lab gains a new tool for making antidepressants, muscle relaxants, or even experimental treatments for neurological disorders.
For people in research, finding practical ways to synthesize or modify these rings matters. Some methods need harsh chemicals, which ratchets up costs and creates nasty waste. Switching to cleaner processes, like using enzymes or lighter solvents, cuts the dangers and keeps budgets from ballooning. Smart labs keep a close eye on these steps, knowing that efficiency doesn’t just mean faster results; it helps get new drugs through regulation and onto shelves.
So much chemistry news focuses on the blockbuster drugs, but the real action often comes from small changes—like adding an –OH group to piperidine. The next time a doctor prescribes a medicine with a weird-sounding name, it’s worth thinking about the careful planning baked into its structure. Behind that prescription, chances are good that compounds like 4-Piperidinol played a quiet but crucial part. Every time researchers build something new out of this basic ring, they set the stage for therapies that could change lives in ways big and small.
Chemistry is full of unfamiliar names, but some substances stand out because they raise questions about health and safety. 4-Piperidinol fits into this group. This chemical shows up most often as a building block for other compounds in pharmaceuticals and industry. Hearing “intermediate” or “precursor” makes its actual role sound kind of distant, but it still packs a punch on its own.
Working around chemicals, especially in a lab or factory, leaves little room for guesswork. Reading the safety data sheets for 4-Piperidinol, something jumps out right away—direct contact irritates the skin, eyes, and respiratory tract. Lab workers who get some of this on their hands are probably heading to the sink pretty fast, because reddening and discomfort follow soon after. If it somehow gets splashed into the eyes, pain and strong irritation happen almost immediately.
It doesn’t just irritate. Animal studies point to more serious effects, such as central nervous system depression, if swallowed or inhaled in significant amounts. Some reports mention headaches, dizziness and even changes in heart function. This isn’t the kind of chemical to treat casually, even during “routine” handling.
Actual danger comes down to concentration and length of exposure. A drop or two on your glove likely won’t end your day, but breathing in dust or vapors—especially day after day—could stack up over time. Most folks who work with this stuff wear gloves, goggles and lab coats. Good ventilation in chemical workspaces keeps inhalation risks low, as fresh air moves out any lingering fumes.
In the pharmaceutical industry, strict guidelines keep things tight. Spills get picked up fast, workers undergo training, and most facilities have emergency showers nearby. Out in less regulated workshops, the risks could rise. Accidents happen more easily where training, ventilation, and safety routines are missing or ignored.
Safety rules often sound tedious until they turn out to be the only thing between a normal day and a trip to the emergency room. Chemical-resistant gloves, eyewash stations, and controlled ventilation all cost money and space, but no shortcut pays off if someone winds up hurt. Even outside the lab, throwing unused chemicals in the trash or pouring solutions down the drain can spark real trouble, either for the environment or for the next person washing dishes.
Proper storage can’t be skipped. 4-Piperidinol needs to stay sealed in tightly closed containers, out of reach of anyone not specifically trained to use it. Labels warn of the hazards for a reason. Good labeling prevents accidents for new and experienced workers alike.
Many chemicals hide behind technical terms and bland descriptions, so it’s easy to skip over the real dangers. Knowing exactly what’s in use, following the right procedures, and staying alert to the risks lowers the odds of injury. If more people took the time to learn what these substances actually do, far fewer would find themselves learning safety lessons the hard way.
4-Piperidinol isn’t famous, but for the workers who mix, bottle or move it from one drum to another, keeping respect for its dangers should always stay front and center.
People often forget how big a role proper chemical storage plays in lab safety. 4-Piperidinol stands as a good example—simple enough to handle with a steady hand, but things can go sideways if someone cuts corners. Experience tells me small details end up saving time, money, and sometimes even health. It’s not just red tape or paperwork; it’s about doing things right from the start.
4-Piperidinol doesn’t announce trouble loudly, but it can stir up hazards. Exposure to air and moisture speeds up degradation. If this stuff finds heat or sunlight, it won’t last long on the shelf and may corrode containers or produce fumes that no one wants to breathe. Leaks from poorly sealed jars can lead to spills or create a stubborn odor that clings for weeks. Keeping this chemical dry and cool isn’t only smart, it stops unnecessary waste.
In my own lab, I’ve seen what works. Use a tightly sealed glass or HDPE bottle. Plastic bags and thin containers just don’t hold up over time; they crack and let in moisture. Stick the bottle in a cabinet away from sunlight, with reliable ventilation. Don’t leave it on a crowded shelf—accidents often happen when someone’s hurrying and knocks over the wrong bottle. For temperature, room temperature usually does the trick, but you really want to avoid any source of heat: radiators, water baths, sunny windowsills. A basic thermometer nearby helps you spot if something’s off before it becomes a costly mistake.
Labels need to be clear, not shorthand nobody will remember three months later. Write the date received and any notes on purity or concentration. Has it been diluted? Was it transferred from the original bottle? These notes matter—especially if you’re working with a busy team. Not every chemical reacts the same way, but 4-Piperidinol won’t play nice with strong acids, oxidizers, or bases. Grouping chemicals by compatibility isn’t some outdated habit. It turns near-misses into a non-issue.
Once a container empties or the expiration date passes, don’t just toss it in the trash. Leftovers demand hazardous waste procedures. Pouring it down the drain is a shortcut that risks both pipes and local waterways. Over the years, regulators tightened their grip on chemical waste for a reason. Following disposal directions may mean spending a few extra minutes logging waste, but those minutes help safeguard everyone who shares the lab.
Everyone has a story about the time a small slip-up made for big headaches. One summer, a bottle sweated onto the shelf because the air conditioning failed. The cleanup took hours. Looking back, a simple insulated container or backup alarm would have made all the difference. Checking bottle caps before closing up for the night, jotting down important details, and organizing shelves—none of this is high-tech, but it gets the job done. Storage guidelines aren’t just rules; they turn out to be the backbone of any safe, well-run lab.
Purity isn't just a fancy number listed on a technical sheet. It’s often a measure of whether a chemical lives up to its promise in your research or process. With 4-Piperidinol, the level of purity will decide how predictable your results look. I’ve worked with batches showing just over 98% purity and others clocking in above 99.5%. Trust me, that fractional difference makes all the difference, especially when you’re standing on the edge of a tricky synthesis.
Most suppliers claim their 4-Piperidinol lands somewhere between 98% and 99.5% pure. That top tier rarely comes without serious attention during manufacturing. Some years ago, I ordered a drum from a lesser-known distributor, chasing a cheaper price. The supposed 99% purity turned out closer to 95% after we ran a third-party HPLC check. Side reactions began sneaking into our workups, adding hours of cleanup and throwing off downstream yields. That’s when the actual stakes of purity hit home.
For pharmaceutical research, even a half-percent impurity can introduce new side products you didn’t bargain for. You don’t want to spend your time tracking down ghosts on the chromatogram or explaining inconsistent data. On the industrial end, that purity gap sometimes means an entire batch must get thrown out because minor unknowns trigger regulatory alarms.
Big claims need hard proof. NMR and HPLC serve as the referees for most lots, with GC checks stretching for volatile traces. You can ask a supplier for a certificate of analysis (COA), but not all COAs deserve blind trust. Anyone working in the lab should feel no guilt sending out fresh samples for third-party confirmation. More than once, our team found unexplained peaks or higher-than-listed moisture. If your project can’t tolerate guesswork, double-checking makes sense.
Plenty of trustworthy companies exist, but the chemical market stays crowded with sellers eager to move product. I learned to stick with reputation over pennies on the price. Chatting with colleagues helps. Their good or bad experiences often reveal which sources cut corners and which ones deliver what’s promised. Buying direct from established players, even if the invoice bites, protects your work and your sanity.
As a buyer, you don’t have to settle for vague data. Push back and ask for spectral evidence, recent purity analysis, or full impurity listings. More suppliers now respond to these requests because smart buyers keep demanding it. In the long run, keeping your standards high encourages the whole supply chain to stay honest. For those tied to demanding research or regulated manufacturing, there’s no game in gambling on unverified purity. Prioritize reliability, insist on clear analytics, and make purity a non-negotiable part of your chemical toolkit.
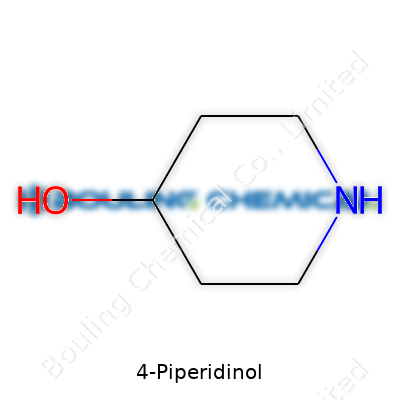
| Names | |
| Preferred IUPAC name | Piperidin-4-ol |
| Other names |
Piperidin-4-ol 4-Hydroxypiperidine 4-Piperidinol 4-Piperidineol |
| Pronunciation | /ˈpaɪ.pəˌrɪd.ə.nɒl/ |
| Identifiers | |
| CAS Number | 626-56-2 |
| Beilstein Reference | 1105071 |
| ChEBI | CHEBI:141564 |
| ChEMBL | CHEMBL31535 |
| ChemSpider | 68252 |
| DrugBank | DB08385 |
| ECHA InfoCard | 100.034.395 |
| EC Number | 202-142-0 |
| Gmelin Reference | 125144 |
| KEGG | C06397 |
| MeSH | D010932 |
| PubChem CID | 98705 |
| RTECS number | TK3150000 |
| UNII | LR60A2IQ5N |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C5H11NO |
| Molar mass | 101.16 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Odorless |
| Density | 1.02 g/mL at 25 °C(lit.) |
| Solubility in water | Soluble |
| log P | 0.44 |
| Vapor pressure | 0.00023 mmHg (25°C) |
| Acidity (pKa) | 10.8 |
| Basicity (pKb) | pKb = 2.84 |
| Magnetic susceptibility (χ) | -7.6 × 10⁻⁶ |
| Refractive index (nD) | 1.485 |
| Viscosity | 18 cP (20°C) |
| Dipole moment | 2.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 274.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -298.2 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4191.7 kJ/mol |
| Pharmacology | |
| ATC code | N07XX08 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS06, GHS08 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 1-2-0-W |
| Flash point | 86°C |
| Autoignition temperature | 235 °C |
| Lethal dose or concentration | LD50 (oral, rat): 700 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 4-Piperidinol: "570 mg/kg (rat, oral) |
| NIOSH | WI9625000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 1-10 g |