In the world of organic synthesis, 4-piperidinecarboxylic acid has been a quiet yet persistent player. Its discovery, stemming from early twentieth-century explorations of nitrogen-containing heterocycles, marked an important step in broadening the toolbox for chemists seeking new scaffolds for drug design and material science. Early work focused primarily on piperidine and its derivatives, leading researchers to realize the value of introducing carboxylic acid functionality directly onto the ring. As methods for piperidine ring construction improved, so did yields and purity of the acid, opening new doors for pharmaceuticals, agricultural chemicals, and analytical research.
This compound shows up as a top choice for building diverse molecular structures due to its unique framework. Both the piperidine core and carboxylic acid group offer reactive sites for countless downstream reactions. In my own research, I’ve seen how 4-piperidinecarboxylic acid provides flexibility—making it much easier to synthesize more complex molecules by enabling both nitrogen and carboxylate chemistry. The acid sits at a crossroads between classic amine chemistry and the rich palette of transformations known for carboxylic acids, which has helped it find a strong foothold in labs focused on medicinal chemistry and chemical biology.
4-Piperidinecarboxylic acid usually appears as a white to off-white crystalline powder. Its molecular weight comes in at 129.16 g/mol, reflecting a structure that’s compact but not overly complex. The melting point hovers in the 191–197°C range, giving it thermal stability valued during purification steps. It displays moderate solubility in water, which increases with temperature, and it often dissolves more easily in polar organic solvents such as methanol or ethanol. The acidic hydrogen, adjacent to a nitrogen atom within the piperidine ring, leads to predictable reactivity that can be leveraged during syntheses involving standard coupling agents or activation strategies routinely used for carboxylic acids.
Laboratories purchasing 4-piperidinecarboxylic acid generally see technical datasheets specifying purity above 98%, with low levels of common contaminants like moisture or residual solvents. Labels always include CAS numbers, chemical formulas (C6H11NO2), batch identification, and clear hazard and handling instructions. From experience, a sealed, moisture-proof container helps avoid caking and degradation. Companies in analytical or regulatory spheres now require full documentation for traceability across quality assurance processes, ensuring that users know the material meets both safety and legal standards.
Industrial production methods rely on catalytic hydrogenation of pyridinecarboxylic acids or by functionalizing simple piperidine derivatives using carboxylation techniques. For those synthesizing at lab scale, starting from 4-cyanopiperidine gives better yields under mild conditions. The cyano group undergoes hydrolysis, typically using strong acids or bases, to deliver 4-piperidinecarboxylic acid. Advances over the years introduced safer solvents, improved catalysts, and procedures that cut down on toxic byproducts, allowing both academic and industrial chemists to scale up with less environmental impact.
The dual reactivity of this compound broadens its use. Chemists can transform the carboxylic acid into amides or esters using carbodiimide coupling or Fischer esterification, paving the way to peptide analogs, functional polymers, or bespoke intermediates. I’ve encountered 4-piperidinecarboxylic acid as a starting point for reductions to the corresponding alcohol or further amidation steps for the production of pharmaceutical building blocks. On the nitrogen, options abound: N-alkylation, acylation, or oxidation let researchers prepare analogs and labeled compounds for mechanistic studies, medicinal leads, or imaging agents in diagnostics. The molecule acts as a workhorse, supporting reactions that would otherwise require multiple starting materials.
Recognized globally, this compound goes by several names, each reflecting its structure in a slightly different way. Some literature calls it piperidine-4-carboxylic acid, while others use gamma-piperidinic acid, correlating with its gamma amino acid backbone. Purchasers may spot trade names or catalog identifiers depending on the supplier. Awareness of these synonyms is more than a bureaucratic quirk—it prevents confusion and ensures chemists select the correct substance, particularly in high-stakes projects like pharmaceutical development or regulatory testing.
Safe use starts with clear respect for the material’s irritant profile. Dust exposure can aggravate the eyes or skin—something every bench chemist has learned the hard way, sometimes only after a careless spill. Standard lab practice includes wearing gloves, safety glasses, and working in a well-ventilated hood. Spill management plans matter as much as inventory logs. Safety data sheets warn against accidental ingestion and prolonged inhalation of fine powders, echoing updated guidance from global agencies like the European Chemicals Agency and OSHA in the United States. Companies and institutions that enforce rigorous chemical hygiene, including regular safety training and chemical inventory audits, see fewer incidents and faster response times to emergencies.
4-Piperidinecarboxylic acid’s reach goes far beyond the chemistry bench. Its presence resonates in the pharmaceutical industry, where researchers use it as a precursor for drugs targeting central nervous system conditions, and in peptide science, where modifications of the piperidine ring anchor novel peptide analogs. Agricultural chemists look toward this scaffold for developing crop protection agents, while materials scientists use its rigid bicyclic shape to engineer polymers with specialized mechanical or conductive properties. Each field draws on the acid’s versatility to chase breakthroughs that shape modern life—from better medicines to more resilient crops.
Every year, patent trackers and academic journals highlight dozens of new uses for 4-piperidinecarboxylic acid. I’ve watched labs deploy it in early-stage synthesis campaigns, moving from benchtop grams to pilot-scale kilograms as candidate drugs or functional materials progress toward commercialization. Bioorganic research has made strong use of its backbone for ring-constrained amino acid analogs—structures that mimic natural proteins yet provide increased resistance to enzymatic breakdown. Whether in life sciences, catalysis, or electrochemical sensing, the acid’s reputation grows with each breakthrough, driven by creative use and solid documentation.
Animal studies and cellular assays guide our understanding of its toxicity profile. Acute effects tend to revolve around local irritation rather than systemic toxicity, with no strong evidence for carcinogenicity or long-term harm at standard exposure levels. Nevertheless, repeated or high-dose exposure can cause headaches, respiratory discomfort, and allergic reactions—a reminder that even familiar compounds require vigilance and respect. Ongoing studies focus on metabolite traces in drug development, with particular emphasis on avoiding unwanted biotransformation or accumulation in patients or workers.
Looking ahead, the demand for 4-piperidinecarboxylic acid is poised to grow, fueled by medicinal chemistry’s ongoing hunt for new scaffolds and the rise of sustainable manufacturing. Researchers are already rolling out biocatalytic routes that generate piperidine acids from renewable feedstocks, cutting down hazardous waste and shrinking energy use. In education, better access to this building block supports advanced training in synthetic techniques and analytical chemistry. If trends continue, next-generation molecules bearing the piperidine carboxy backbone could show up in targeted cancer therapies, biodegradable materials, or smart biomolecule sensors that monitor health in real time.
In the world of chemistry, some compounds never make headlines, but they work quietly behind the scenes, powering entire industries. 4-Piperidinecarboxylic acid stands as one of these unsung tools. To most people, the name may look like a spelling bee puzzle. For chemists, pharmacologists, and manufacturers, its uses reach well beyond the blank stares its name might invite.
The pharmaceutical industry draws heavily on molecules designed to interact precisely with the chemistry of the human body. 4-Piperidinecarboxylic acid isn’t one of those miracle pills on a drugstore shelf, but plays a central role as a building block in many drug synthesis pathways. Scientists often use it to create treatments for conditions such as epilepsy and Alzheimer’s disease. For instance, it serves as a core component in some anti-epileptic medications by helping to produce key intermediates that eventually get converted into active drugs.
The reason this compound gets such attention has to do with its ring-shaped molecular structure. It interacts predictably in reactions, giving chemists a kind of reliability that’s not always easy to find in raw materials. That reliability helps keep drug development safe and predictable. You need compounds that react as expected, every single time. Otherwise, you’re rolling the dice with medicines, which is never a good plan for public health.
Academic labs and commercial operations both count on this acid for research projects. It works as a scaffold—think of it as the backbone—of more complex molecules. Researchers at universities often tweak its side groups to see what happens to biological activity. Some variations open doors to new treatments for neurological diseases or pain relief options stronger and safer than existing products.
Beyond direct use in medicines, companies hire this compound for creating specialized intermediates. Those intermediates then get sold to other firms for a wide range of uses, from agrochemicals to fine chemicals. Some flavors and fragrances even trace their chemistry back to building blocks that started life as 4-piperidinecarboxylic acid.
Trust in these chemicals begins with purity and consistency. Dirty batches can throw off experiments or, worse, introduce unknown hazards into medications. Factories producing the acid stick to rigid checks. The United States Pharmacopeia (USP) and similar organizations set strict requirements for acceptable purity, keeping companies honest and giving regulators confidence that what's being handled in research and production matches global safety standards.
Reliable chemical sources rarely come cheap. Research teams, pharmaceutical firms, and even some startups wrestle with high costs for guaranteed quality. Disruptions hit hard during shortages, highlighting the importance of supply chain resilience. Domestic production and global partnerships, aimed at easing bottlenecks, have become a focus for chemical suppliers, especially after pandemic-era delays.
As science pushes boundaries, new therapies and materials hungry for advanced chemicals emerge each year. The onus falls on suppliers and policymakers to weigh not just business or research interests, but also long-term safety and environmental impacts. Reducing waste, improving manufacturing techniques, and developing green chemistry alternatives can help ensure that progress also supports public health and the planet.
Sitting at the core of 4-Piperidinecarboxylic acid is a six-membered ring, called a piperidine ring. This ring has five carbon atoms and one nitrogen atom. At the fourth carbon, a single carboxylic acid group pokes off the ring. Its chemical formula is C6H11NO2. The structure might seem basic on paper: a cyclic amine with a carboxyl group attached to it. In practice, even a minor change in the position of that carboxyl group can produce a compound with completely different properties.
Picturing 4-Piperidinecarboxylic acid in a laboratory notebook, you’d see a hexagonal ring. The nitrogen atom forms part of that ring, and at the fourth carbon, a COOH group pops out. You’re looking at a molecule combining the properties of an amine and a carboxylic acid. Both of these groups participate in reactions common in pharmaceuticals and peptide chemistry. In research, precision in drawing and thinking about this structure changes outcomes. Put the carboxyl group on the second or third carbon, and you’re not talking about the same chemical anymore.
Working with compounds in the piperidine family, one habit grows solid—always double-check the arrangement of atoms. Ring systems such as piperidine show up in drugs, especially those targeting the nervous system. The exact position of a carboxylic group determines not only the type of reactions possible but also how stable or reactive the compound becomes under different conditions. Even things like solubility in water or the melting point shift with a change in structure.
Those doing medicinal chemistry see these kinds of acids turn up as building blocks or intermediates. The acid’s amine ring offers a place for additional chemical tweaks, giving scientists room to craft new molecules for treating everything from infections to mental health issues. As a hands-on example, I once worked in a lab exploring small molecules for neurology research. Moving a single functional group on a ring system changed the potency of our molecules by orders of magnitude.
Mislabeling or misunderstanding chemical structure can collapse entire research projects. Every atom and bond must show up in the right spot, especially for chemicals involved in drug synthesis or biological pathways. Getting it wrong invites confusion and wasted funding. Researchers rely on accurate molecular information—such as the structure of 4-Piperidinecarboxylic acid—to design better experiments and predict results. In medicine, purity and structure literally become a matter of safety.
Documentation and verification matter. Reliable labs use nuclear magnetic resonance (NMR) spectroscopy or mass spectrometry to prove that the structure matches expectations. If someone skips these steps, shortcuts often come back to bite. In the pharmaceutical world, regulators demand a detailed accounting of every bond and group in a drug candidate’s structure before any approval is granted. Peer-reviewed literature builds trust by sharing detailed analytical data for chemicals like this one. That’s how science earns its credibility.
Researchers can cut down on errors by adopting tools that check for structural accuracy before a synthesis even begins. Software and databases have grown advanced, sometimes flagging subtle differences between similar molecules. It pays to share analytical data widely. Mistakes do less harm when caught early, and sharing best practices strengthens the reliability of chemical research. Success in lab work begins with small details—like remembering that 4-Piperidinecarboxylic acid puts its carboxyl group on the fourth carbon, not anywhere else.
4-Piperidinecarboxylic acid turns up in dozens of chemistry labs and production plants. Whether research teams or manufacturers order it, questions about purity always emerge. Looking at the range of available purity grades helps illustrate how chemical sourcing plays out in real-world settings, not just on specification sheets.
In my experience with buying chemicals for both research and consultancy, purity isn’t just a number that sits on a technical data sheet. Lower purity grades sometimes bring a mixture of related compounds, batch residues, or moisture content. For organic chemists, even a trace contaminant can tank a project or skew experimental data. Pharmaceutical labs keep a wary eye on each impurity, worried about unexpected toxicity or altered bioactivity.
When a supplier lists 4-piperidinecarboxylic acid as “technical grade” or “lab grade,” they’re signaling differences that have more than accounting consequences. Technical grade suits industrial synthesis where a compound might go through further purification steps. Academic and pharmaceutical settings often demand “analytical” or “high-purity” material—sometimes 98% or higher—just to make sure that downstream reactions behave as predicted, or to meet regulatory standards.
Procurement isn’t a one-size-fits-all affair, and just because a technical grade exists doesn’t mean it works everywhere. I’ve watched grad students pick up bargain solvents thinking all acetone is equal, only to watch their yields drop through the floor. It’s the same with 4-piperidinecarboxylic acid—cutting corners on purity sometimes looks cheap and convenient but comes back around as lost time and faulty results.
Regulatory authorities like the FDA and EMA take purity issues seriously, especially if a compound could end up in a drug formulation. Residual solvents, metals, or process byproducts can trigger recalls and damage trust. Academic journals lose patience with “gray area” data, too—if you can’t prove your compound’s purity, your publication prospects take a hit.
A key part of working with chemical suppliers has meant asking the awkward questions: what impurities appear, what detection methods did they use, how often do batches get checked? Genuine suppliers don’t mind handing over certificates of analysis or discussing their controls. Fly-by-night shops usually dodge the issue or flash a price too good to be true.
Traceability isn’t just industry jargon; it protects end users and promotes honest competition. It only takes one poorly characterized batch to learn the hard way: saving on materials rarely means saving in the long run.
Solutions do exist. Researchers can partner directly with reputable distributors, tap into transparent marketplaces, and insist on certificates verifying analytical methods—not just purity percentages. For larger companies, auditing suppliers periodically weeds out weak links.
Academics fare best by requesting small samples and running their own quality checks before full-scale experiments. More sharing about failed syntheses, rather than sweeping them under the rug, pushes labs and suppliers toward higher standards across the board.
All in all, purity levels in 4-piperidinecarboxylic acid and related compounds shape research and industry outcomes at nearly every step, sparking strong opinions from anyone who’s depended on consistent supplies.
4-Piperidinecarboxylic acid isn’t a household name, but anyone working with specialty chemicals knows that a lapse in storage protocol can leave you in a difficult spot. This compound holds significance in the pharmaceutical and chemical industries due to its uses in designing drugs and other fine chemicals. Every researcher and technician has encountered that messy stockroom, where the lack of storage rules led to ruined supplies and, sometimes, a hazardous cleanup.
Heat, moisture, and air play havoc with many organic acids, and 4-Piperidinecarboxylic acid isn’t immune. Exposure to water or humid air can push it to break down or become less pure—an expensive lesson for anyone juggling tight budgets or deadlines. Contamination from neighboring chemicals or sunlight can bring on chemical changes. Products with compromised purity don’t just risk a failed experiment; they threaten health and safety for anyone handling the material.
Dryness isn’t just a suggestion here. I always rely on airtight containers, preferably ones made of glass or high-quality plastic, to cut off moisture and air. Chemical storerooms can see wild temperature swings, so climate control tells the difference between a stable compound and a container full of wasted product. Room temperature often works, but storing in cool spaces and away from direct sunlight shields against accidental warming or exposure. Shelving in a dedicated cabinet keeps 4-Piperidinecarboxylic acid separated from strong acids, bases, or oxidizing agents—a lesson reinforced by more than one near-miss in crowded labs.
A clear label is never overrated. Each bottle should spell out the full compound name, date received, and concentration, along with a hazard warning. Sloppy handwriting or missing dates invite mistakes and search time. Inventory logs or digital spreadsheets, updated by whoever handles stock, lower the chance of overlooked hazards or expired containers. Routine checks guarantee that aging stock doesn’t slip through the cracks and end up spoiling a large batch of raw material.
During my years in the lab, an unlabeled bottle once left us guessing as to its contents, wasting hours and raising risk. Not everyone sees the immediate danger in sloppy storage until they have a close call. Gloves, goggles, and lab coats block skin and eye exposure if accidents happen. Chemical burns or inhaled vapors have real health costs. Knowing where the material safety data sheet sits—and taking two minutes to read it—beats learning from regret.
Storage doesn’t end at keeping chemicals in a cupboard. For larger stocks, desiccators with silica gel packets give extra moisture control. Locking chemical cabinets set apart acids and bases, cutting the odds of a dangerous mix. Institutions that invest in staff training and regular audits find fewer incidents than those without clear policies. Automated inventory keeps everyone on the same page. Taking time now to build solid habits shields workers, equipment, and the bottom line.
Proper storage of 4-Piperidinecarboxylic acid protects quality and keeps people safe. Experience shows that cutting corners with chemical storage gains nothing. The facts and real-life lessons validate the extra steps. Spend the time, check your labels, and store smart—future you will thank you.
Anyone who works in a lab recognizes the value of taking chemical hazards seriously. 4-Piperidinecarboxylic Acid isn’t one of those substances found under the kitchen sink; it pops up more in organic synthesis and pharma research. Its risk comes not from explosive drama but rather from what you don’t always see: skin, eye, and respiratory irritation, and the slow build-up of bad habits leading to accidental exposure.
Splash-resistant goggles matter more than regular reading glasses. Even a quick pour can kick up droplets or fine dust. I’ve watched colleagues ignore this, only to blink through a whole afternoon because of a stinging eye. A solid pair of nitrile gloves makes a big difference, especially when handling powders. Short sleeves and open shoes won’t cut it; skin protection starts with a buttoned lab coat, closed footwear, and gloves that fit. Chemical burns can sneak up—that red patch on your wrist proves it.
Fume hoods aren’t just bulky furniture; they handle vapors and dust that could land in your lungs. Respiratory effects creep in if you get careless. Lean into the hood, minimize open bench use, and avoid talking or turning away in the middle of pouring or weighing. If local exhaust is missing, it’s time to rethink whether to proceed until proper controls are in place.
Quick cleanup matters much more for powders like 4-Piperidinecarboxylic Acid. Dry material on the bench disperses and makes contact easier. A simple brush can toss the dust into the air, increasing the risk. Wet wiping with appropriate decontaminants or absorbent pads keeps everything controlled. Always check that the spill kit is restocked—safety supplies are only useful if they’re on hand. Avoid making up procedures in the moment; rehearsed steps keep panic at bay.
Clear labeling prevents confusion. Handwritten notes fade; proper labels with chemical names, concentrations, and hazards never blend into the background. Store 4-Piperidinecarboxylic Acid away from incompatible substances and water-sensitive chemicals. Even in a busy lab, order matters—grabbing the wrong bottle wastes time, but picking the wrong chemical can ruin your day.
No one walks in already knowing good chemical hygiene. Early in my career, I skipped steps out of impatience, but there’s no shortcut to careful technique. Practical training, not just safety sheets, helps develop safer habits. Drills about spills and first aid drive real learning, making the correct response automatic.
Blaming someone for a spill or minor contact with 4-Piperidinecarboxylic Acid doesn’t stop future slips; open communication fixes process gaps. Every exposure, even minor, deserves documentation and review. I’ve seen small reports lead to big improvements, like updated goggles or better fitting gloves.
Creating a safe workspace feels tedious until the day something goes wrong. You start appreciating the checklists, the regular PPE checks, and the buddy who double-checks procedures. Good safety practices turn potential disaster into just another day at work.
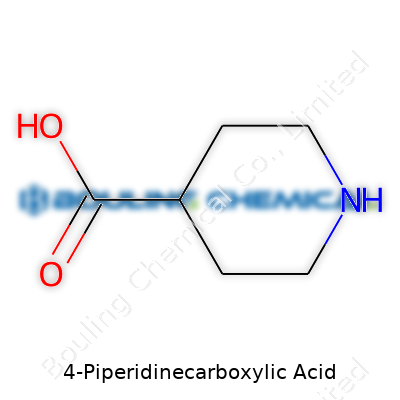
| Names | |
| Preferred IUPAC name | Piperidine-4-carboxylic acid |
| Other names |
Piperidine-4-carboxylic acid Isonipecotic acid Isonipecotate |
| Pronunciation | /ˈpɪp.əˌrɪd.iːn.kɑːrˈbɒk.sɪl.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 499-02-5 |
| Beilstein Reference | 82834 |
| ChEBI | CHEBI:89432 |
| ChEMBL | CHEMBL16328 |
| ChemSpider | 16114 |
| DrugBank | DB04225 |
| ECHA InfoCard | DTXSID9020828 |
| EC Number | 1.3.1.83 |
| Gmelin Reference | 102107 |
| KEGG | C06125 |
| MeSH | D010896 |
| PubChem CID | 70104 |
| RTECS number | UG3675000 |
| UNII | 18FW1V92WU |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID6021167 |
| Properties | |
| Chemical formula | C6H11NO2 |
| Molar mass | 129.17 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.2 g/cm3 |
| Solubility in water | soluble |
| log P | -1.4 |
| Vapor pressure | 0.0000133 mmHg at 25°C |
| Acidity (pKa) | pKa = 10.75 |
| Basicity (pKb) | 2.87 |
| Magnetic susceptibility (χ) | -41.5 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.498 |
| Dipole moment | 3.07 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 142.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -331.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2449 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319 |
| Precautionary statements | P261, P264, P270, P271, P272, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 151°C |
| Lethal dose or concentration | LD50 oral rat 5700 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2150 mg/kg (rat, oral) |
| NIOSH | SN3500000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.03 mg/m³ |
| Related compounds | |
| Related compounds |
Proline Nipecotic acid Isonipecotic acid Piperidine Pipecolic acid |