Digging back through the pages of chemistry, 4-Nitroimidazole first grabbed real attention in the late part of the twentieth century. Scientists searching for new medicinal scaffolds stumbled upon the broader imidazole family, then started tinkering with the imidazole ring by adding a nitro group at the 4-position. This small chemical twist set the tone for decades of research, particularly after folk noticed its potential in treating parasitic infections and its use as a marker in hypoxia research. It stands as a patient product of dedication, trial, and—let’s be honest—lots of time spent in stuffy labs hovering over reaction vessels and old glassware.
4-Nitroimidazole shows up as a pale yellow powder, catching the eye with its distinct color. Chemists and pharmacists alike know it as a reactive backbone for new drug development. It’s found a role in everything from pharmaceuticals to biochemical assays. In its pure form, a jar of this compound sits on laboratory shelves, labeled with caution and potential, waiting for the next chapter—be that animal studies or purified drug batches.
This powder isn’t the friendliest of customers: it doesn’t like water, dissolves more willingly in some organic solvents, and starts to decompose if you heat it too long. It offers a melting point somewhere between 193–198°C, allowing chemists to verify its purity. The nitro group imparts both electron-withdrawing tendencies and reactivity at specific sites, giving this molecule a temperament that suits laboratory transformations but demands respect from any handler.
Manufacturers put information on labels that matter to every user: grade (analytical, reagent, pharmaceutical), batch number, purity, recommended storage temperature, and shelf life. Safety warnings jump out—harmful if inhaled or swallowed, irritating to the eyes and respiratory tract. I’ve seen too many young lab techs glance over these warnings; after a stern word from a senior chemist, nobody makes that mistake twice. Part of that is experience; part of it self-preservation.
Chemical syntheses of 4-Nitroimidazole have streamlined over the years. Earlier routes got tangled up in side-products and questionable yields. These days, people start with glyoxal and ammonium acetate, introducing a nitration step—often using nitric acid under careful cooling and constant stirring. Depending on the method, microwave-assisted approaches and green chemistry tweaks can limit waste. Even so, handling concentrated acids in a fume hood still gives most chemists a good reminder: safety goggles aren’t optional.
If you hand 4-Nitroimidazole to someone with an organic synthesis background, creative modifications start churning. The nitro group’s electron-withdrawing effect lets the molecule absorb reduction reactions with sodium dithionite or catalytic hydrogenation, producing amines that open up channels to more complex pharmaceuticals. Halogenation, alkylation, and cross-coupling provide further options, making this a sturdy base for research into anti-infective and anticancer compounds. Seeing how chemists poke and prod this structure gives you a sense of just how versatile small molecules can get.
Shopping for chemicals requires knowing all aliases. 4-Nitro-1H-imidazole, NSC 15188, and Imidazole, 4-nitro- all refer to the same compound. The diversity in names sometimes causes confusion, especially among students toggling between supplier catalogs and journal articles, but careful checking avoids costly mistakes. Every veteran researcher has a story about a mislabeled package or a wrong chemical in a bottle.
No shortage of caution surrounds this compound. Laboratory safety protocols demand gloves, respirators, and goggles during weighing, dissolving, or transferring. Direct contact means trouble for skin or airways—its toxicity profile makes that plain. Chemical storage means dark bottles in cool, non-humid cabinets. Local occupational practices call for handling under fume hoods, with spill plans tested every year. Accidents remain rare, but only because chemists drill procedures and cultivate a culture of caution. The phrase "a little paranoia never hurt anyone" often echoes through the corridor during lab safety meetings.
Medical research snapped up 4-Nitroimidazole almost as soon as its antimicrobial properties showed up. It plays a role in targeting anaerobic bacteria and protozoa, while radiolabeled derivatives trace hypoxic regions in cancer diagnosis and research. Agriculture eyes it for parasite control in livestock, although regulations restrict its use in food animals in several countries due to safety concerns. Environmental science also tests its transformation and persistence, fueling studies into soil and water remediation.
Development efforts keep this molecule in the conversation. Pharmaceutical companies explore its derivatives for new therapies, targeting infections where older drugs fail. Using 4-Nitroimidazole as a template, research teams across the globe have spun off countless analogs, hunting for better specificity, reduced toxicity, or more favorable metabolic profiles. Competition between labs sometimes leads to secrecy, but the steady stream of papers and patents proves nobody’s resting easy. Collaborations around synthesis improvements and bioassay screening move things faster, especially with new diseases cropping up.
Every time a compound advances toward medical use, toxicity research ramps up, often revealing trade-offs between activity and safety. 4-Nitroimidazole shows both promise and risk; animal studies chart neurotoxic effects and organ exposure at high doses, and chronic dosing studies examine long-term effects. Regulatory agencies scrutinize data before approving broader use, which has limited recreational or agricultural expansion. In my own experience, toxicology reports sometimes get dismissed as afterthoughts by eager researchers, but ignoring this step takes its toll—no one wants to recall a drug or see an environmental hazard story tied to their lab.
Where does this molecule go next? Scientists look to tweak the core structure and attachment points, hoping to develop next-generation drugs with fewer side effects. Regulatory trends steer compound developers toward safer, greener synthesis methods. The digital age brings modeling and prediction tools to the bench, letting chemists virtually test analogs before hitting the lab. Collaboration between industry, government, and academia will play a role; the field rewards those who think beyond the boundaries of one discipline or lab bench. While challenges lie ahead, especially with safety assessments and regulatory scrutiny, those challenges feed progress and innovation—so the story of 4-Nitroimidazole stretches on, fueled by persistent curiosity and a steady hand at the bench.
4-Nitroimidazole, a simple ring-shaped compound, has found its place in science labs around the world. Despite the name sounding intimidating, it’s not some mysterious chemical with a single hidden purpose. I first spotted this name in a drug development paper in college. It looked like another forgettable molecule, but the backdrop around it told a very different story.
Oncologists often wage a relentless battle with cancer. Tumors, especially the aggressive ones, develop hypoxic (oxygen-poor) zones where most drugs struggle to work. 4-Nitroimidazole steps in as a radiosensitizer, making hard-to-treat tumors more vulnerable during radiation therapy. Nitroimidazoles latch onto hypoxic tumor cells, helping radiation therapy do its job more effectively. That’s a big win for stubborn tumors and for cancer patients facing tough odds. A lot of research since the 1970s has backed up this role—showing increased cancer cell kill rates, fewer relapses, and in some cases, a boost in overall survival.
Doctors and patients benefit when treatment options become more flexible and potent. With the right dose and monitoring, 4-Nitroimidazole carries the potential to tip the scales in favor of successful therapy. Not every patient needs it, and not every tumor will react the same way, but having this tool in the arsenal is reassuring.
It isn’t about cancer alone. Microbiology labs use 4-Nitroimidazole as a building block for other, more complex compounds. Hundreds of drugs owe their origins to tweaks made on imidazole rings. Antibiotics, antiparasitics, and antiprotozoals start with molecular fragments like this as the cornerstone. Its derivatives show up in frontline treatments against infections such as Giardia, Trichomonas, and amoebiasis.
During my time working in a university chemistry lab, we would synthesize variants of nitroimidazoles, testing their antibacterial power. Sometimes a small change at the molecular tail led to a compound with remarkable activity against tough bacteria. Local hospitals appreciated those samples—especially when resistant bugs made headlines. Many times, it’s the under-the-radar chemicals like 4-Nitroimidazole that quietly fuel both breakthroughs and everyday pharmacy shelves.
A tool this powerful deserves respect. Studies show 4-Nitroimidazole can be toxic at high doses, especially for the nervous system and reproductive organs. Scientists who work with it always use gloves, goggles, and proper ventilation. No one wants to take shortcuts when side effects can range from mild headaches to nerve damage. I’ve seen researchers become complacent after years in the lab—only for one spill to remind them why those safety rules matter.
Science never stays still. Newer analogues based on the 4-nitroimidazole framework are getting attention for their promise against drug-resistant infections and as more targeted cancer agents. Research funding and collaborative trials make this possible, alongside responsible regulation to monitor risks and side effects. More detailed studies and real-world feedback guide safer, smarter use.
In the bigger picture, 4-Nitroimidazole stands as a reminder of how a humble molecule can connect lifesaving cancer care, infectious disease treatment, and cutting-edge research. Whenever new students ask why we fuss over plain-looking chemicals, I point to stories like this. Unassuming at first glance, but essential in the hands of doctors, researchers, and patients alike.
4-Nitroimidazole shows up in a lot of research labs and some manufacturing sites. Before dealing with this compound, I always start with skin protection. Nitrile gloves keep the chemical off my hands, especially since it can irritate the skin and may get absorbed. Goggles are non-negotiable—fumes and splashes can take your vision fast, and most of us working in labs have had at least one near miss. A lab coat or gown goes on each time, buttoned to the neck, so sleeves and shirts stay clean and nothing soaks through during long procedures.
Breathing 4-Nitroimidazole dust or vapor can mess up your lungs over time. I always choose a workspace with a running fume hood, not just a cracked window or a desktop fan. Good airflow pulls vapors away before they reach your nose. Some folks skip this step, thinking the stuff is “not that volatile.” Trouble is, even a low concentration over many days can build up in the body. That’s the kind of slow harm you don’t feel until it’s too late.
Spills do happen, usually when folks rush or fill containers too full. Absorbent pads or spill kits need to sit close by—trying to grab those across the room wastes precious seconds. Once a substance like 4-Nitroimidazole hits a bench or floor, you can’t just wipe it with paper towels or flush it down the sink. Chemical-resistant gloves and a mask go on, and the pads go down right away. Everything soaked with the chemical goes into a labeled hazardous waste bag, which our facility stores far from break rooms and busy corridors.
In my experience, accidents often trace back to poor storage. 4-Nitroimidazole stays stable in a cool, dry place, away from direct light and heat. I keep it on a low shelf, inside a tightly sealed container, and nowhere near acid or base bottles—mixing those could trigger a reaction, and nobody wants to evacuate the whole building over a shelf mistake. Clear labeling is simple but crucial, since plain bottles often lead to confusion during busy projects or shift changes.
If some of the chemical splashes on your skin, rinse with running water right away—there’s no upside to “waiting until you're done.” For eyes, hit the eyewash station and keep flushing for fifteen minutes. I’ve seen a few folks want to tough it out; that always backfires. Get checked by medical staff without delay. With inhalation, get outside fast and call for help. It’s easy to underestimate chemical fumes, but headaches and shortness of breath are trouble signs.
Folks sometimes make lab safety sound overcomplicated. I find that routines work better than fancy posters. Using gloves, goggles, and hoods every time keeps me from cutting corners. Waste bags everywhere remind everyone to toss contaminated gear right away. Staff training works best when people walk through a spill drill, actually put on PPE, and see how fast the right tools can fix a mess.
People learn to be careful by watching coworkers. New hires tend to copy what old-timers do. Leading by example protects everyone, even during tough deadlines. Keeping 4-Nitroimidazole out of break rooms, using separate waste bins, and checking containers each week aren’t just extra chores—they help everyone go home healthy at the end of the day. The extra effort repays itself because there’s no shortcut that’s worth an accident you can't undo.
4-Nitroimidazole isn’t a household name but it pops up in labs and under the hoods of researchers’ benches. Once, I found myself sorting through a shelf lined with chemicals, labels faded, caps crusty. You notice quickly how easy it gets to overlook something that can't shout at you. This compound isn’t something to toy with—the nitro group alone gives away enough about reactivity and safety concerns.
People might think storing chemicals is just about sticking bottles on a shelf, but every time I’ve seen someone ignore the basics, bad things happened. No point risking your health—or the building—over a small shortcut. For 4-Nitroimidazole, a tight container always makes sense. Don’t leave powder or bits lying in a box or a sachet. Find a screw-top bottle, something that won't crack if dropped, and that seals out the air.
The thing with nitro compounds: heat and moisture don’t do them any favors. High school chemistry drilled into me how quickly some organics degrade or, worse, become hazardous. A cool cupboard works. No spot near heating ducts, sunlit windows, or above stoves. Think below room temperature. A desiccator helps if you live somewhere muggy; humidity eats away not only at the compound, but often at your peace of mind too.
A legible, detailed label always helps months later, when the memory of what you put away starts to fade. Include the date received, who handled it last, and any hazard not obvious from the name alone. I once dodged a headache thanks to a bold “toxic by inhalation” note scribbled across old masking tape. Clear labeling saves time and, some days, your skin.
Certain compounds don’t play well with others. Oxidizers, acids, reducing agents—put some room between your 4-Nitroimidazole and the rest. Separate shelf space works, never above or below something reactive. I’ve watched containers sweat and crystalize during hot summers. Keeping space between chemicals reduces accidental contact if a spill or bottle crack happens.
Not everyone who steps into a workplace or storage room knows chemistry. I’ve walked into break rooms where a bottle left open created confusion and real danger. Always keep 4-Nitroimidazole stored in a locked cupboard or behind a closed door. Make sure only trained folks get access.
No plan lasts forever—spills and accidents creep up, no matter how careful you stay. Stock absorbent pads, gloves, and goggles right where you store the chemical. Know your emergency contacts and have the chemical’s safety data sheet handy. During a small spill at my old job, the quick grab of a spill kit and a copied procedure on the wall kept things under control.
Good records and regular checks keep small problems from growing. Look at containers monthly, log usage, and check shelf dates. The earlier you spot crusty residue or corrosion, the easier your life gets. Storing 4-Nitroimidazole isn’t guesswork, but it rewards anyone willing to give it respect.
A bottle stamped with “4-Nitroimidazole” might seem simple, just another compound sitting on a shelf. Peel back the label, though, and purity becomes the real headline. Research labs, pharmaceutical developers, and fine chemical suppliers treat that purity number as make-or-break. I remember one project nearly thrown off-course by a batch with an off-spec impurity, barely a fraction of a percent – ruined hours, lost resources, and a round of sheepish phone calls.
Most reputable chemical suppliers specify 4-Nitroimidazole at a minimum purity of 98%. Others push it to 99% or even higher, purely responding to the tension between price and application. Trace byproducts like imidazole, 2-nitroimidazole, and a dusting of moisture can trip up synthesis, especially in medicinal chemistry. These background players might sound harmless, but in a reaction flask, they’re troublemakers. For regulated applications, say in active pharmaceutical ingredients, that extra percent changes everything. One NMR spectrum or HPLC trace will light up every impurity, and those peaks spark debates over reorders, requalification, and sometimes, full method development reruns.
No one wants to hear about dull lab days turned disaster. Pure 4-Nitroimidazole, on paper, should get the job done every time. My own projects taught me purity shortcuts always wind up costing more. Lower grade material eats up more solvent during recrystallization. Byproducts gum up glassware and tempt fate on yield losses. You pay for every point below 99% with troubleshooting hours. In a recent scale-up, one team at a contract lab told me they almost missed a deadline because the incoming “lab grade” batch turned out to be barely 95%, stuffed with hard-to-remove cousins. They had to run cumbersome cleanups, order another lot, and explain the mess on a client call.
Suppliers might hand over a neat certificate of analysis, detailing purity, identification, and water content. Still, smart labs pull out their own HPLC, titration, or melting point tests for every new batch. Sometimes I see spectroscopists swapping stories in lunch rooms about unexpected UV traces or discoveries lurking in the end of the chromatogram. The best way forward always means setting internal benchmarks – don’t trust a vendor blindly, establish a habit of double-checks. Even the labs with the best reputations sometimes get batches wrong, whether from human slip-ups or differences in test methods.
Manufacturers could help by publishing full impurity profiles with every batch, not just the headline purity figure. Investing in customer feedback channels makes a difference, too. I’ve seen companies work wonders after hearing complaints about a mysterious odor or color shift, probing deeper into their cleaning or drying steps. A chain of trust only stays strong when every link – chemist, supplier, and client – takes accountability for their piece. In this race for cleaner, safer, and more reliable material, transparency makes science work better for everyone.
Someone asking for the SDS for 4-Nitroimidazole isn’t just ticking a box. Often, requests like this start at the workbench of a lab tech, a shipping dock worker, or a quality control officer who wants to make sure the right steps get taken before the next bottle is opened. People don’t ask for these documents out of curiosity—it’s usually the plain reality that no one wants to breathe something hazardous, spill it on their arm, or find out too late that a chemical can burn through gloves in seconds.
I remember a time in a small college lab when a misplaced bottle with a faded label led to a mad scramble for the SDS. We didn’t have it on file, and everyone ended up standing around the fume hood, holding smartphones and digging through dubious websites. In the end, we had no idea what spilled on the countertop. Uncertainty meant risking someone’s health. It would have taken one up-to-date SDS to prevent that chaos.
Stories about chemical exposure don’t get much attention unless they’re spectacular, but plenty of workers quietly suffer from careless handling and missing data. The SDS is often the only resource standing between someone and a regrettable accident. This sheet explains, in plain language, what’s flammable, what reacts violently, the right kind of gloves, the proper way to breathe around the substance, and basic first aid. Even something that seems minor—like knowing 4-Nitroimidazole gives off irritating fumes when heated—makes a difference.
It’s not just science professionals who ask for this data. Cleaning teams, warehouse workers, and transport drivers often face chemicals either during deliveries or by handling waste. With more substances flowing through the global supply chain, the risks multiply fast when clear instructions get buried in bureaucracy or a password-protected vendor portal.
Even in 2024, tracking down a real, current SDS for specialty chemicals isn’t a given. Plenty of sites claim to host these documents and yet provide broken links, outdated information, or paywalls. For anyone outside a well-run company, hunting for this sheet means jumping through hoops that might lead to guessing—or making mistakes. That’s the real issue: lack of open access leads to risks nobody intended.
One honest fix starts with open-access databases that don’t just dump a scanned PDF, but make the latest version easy to search and download. Think of what a difference it would make if, like nutritional facts on food packaging, every chemical needed a small, readable QR code on its label leading straight to the right SDS in several languages. This effort would save phone calls, wasted time, and maybe even a trip to the hospital.
In the end, requests for an SDS are about showing respect for those who handle substances every day. The call for an updated sheet represents a need for transparency—nothing fancy, just a fair shot at going home healthy. Companies who keep this information up to date, store it where people can find it, and walk everyone through the details are making a big difference. Having worked jobs that depended on fingernail-level details, I can tell you that paperwork matters a lot more when you realize your skin and lungs might pay the price later on.
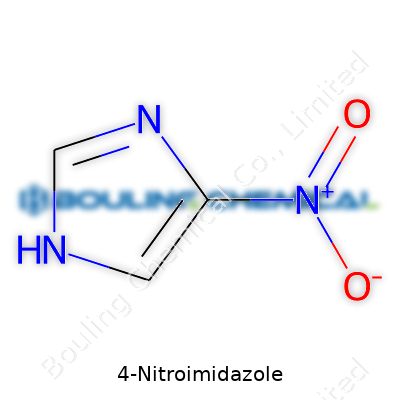
| Names | |
| Preferred IUPAC name | 4-nitro-1H-imidazole |
| Other names |
4-Nitro-1H-imidazole 4-nitroimidazol |
| Pronunciation | /ˈnʌɪ.trəʊ.ɪˈmɪd.əˌzoʊl/ |
| Identifiers | |
| CAS Number | 3034-38-6 |
| Beilstein Reference | 120918 |
| ChEBI | CHEBI:5170 |
| ChEMBL | CHEMBL41586 |
| ChemSpider | 81638 |
| DrugBank | DB00212 |
| ECHA InfoCard | 201-776-4 |
| EC Number | 2.5.2.3 |
| Gmelin Reference | 79057 |
| KEGG | C07314 |
| MeSH | D009605 |
| PubChem CID | 13915 |
| RTECS number | QR0525000 |
| UNII | R3D8U6NP3N |
| UN number | UN2661 |
| CompTox Dashboard (EPA) | 4-Nitroimidazole: "DTXSID5120992 |
| Properties | |
| Chemical formula | C3H3N3O2 |
| Molar mass | 114.09 g/mol |
| Appearance | Yellow to light brown solid |
| Odor | Odorless |
| Density | 1.45 g/cm³ |
| Solubility in water | Soluble |
| log P | 0.02 |
| Vapor pressure | 3.07E-5 mmHg at 25°C |
| Acidity (pKa) | 9.43 |
| Basicity (pKb) | 11.90 |
| Magnetic susceptibility (χ) | -63.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5660 |
| Dipole moment | 3.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 204.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -27.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1110 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | J01XD02 |
| Hazards | |
| GHS labelling | GHS02,GHS07 |
| Pictograms | GHS07,GHS08,GHS09 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P305+P351+P338, P304+P340, P312 |
| NFPA 704 (fire diamond) | 3-2-1-* |
| Flash point | 104°C |
| Autoignition temperature | 529°C |
| Lethal dose or concentration | LD50 (oral, rat): 960 mg/kg |
| LD50 (median dose) | LD50 (median dose): 960 mg/kg (rat, oral) |
| NIOSH | TTD6596000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 25°C |
| Related compounds | |
| Related compounds |
Imidazole 2-Nitroimidazole 5-Nitroimidazole Metronidazole |