Chemistry has always operated on the back of small discoveries that quietly fuel entire industries. 4-Morpholinopiperidine came out of research during the 20th century as scientists tried to build more versatile organic building blocks for pharmaceuticals and agrochemicals. Chemists explored rings that would deliver stability, polarity, and unique reactivity, and piperidine derivatives with morpholine rings answered that call. The path from basic piperidine compounds to this heterocycle didn't happen overnight; it moved through breakthroughs in nitrogen ring chemistry, where each tweak added some new property. Those early years, much of the work happened behind closed laboratory doors, in university labs and research centers, with patents pointing toward its promise as both an intermediate and as a scaffold for future drug work.
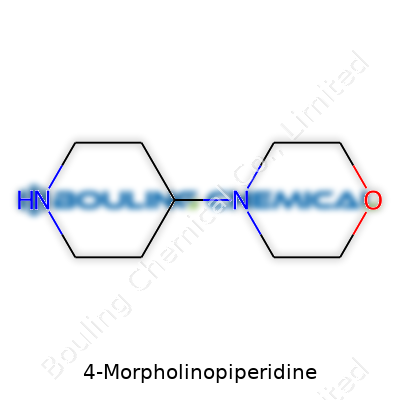
4-Morpholinopiperidine stands as a piperidine ring fused to a morpholine ring, linking two six-membered structures, both loaded with nitrogen. It lands on shelves as a white, often crystalline solid, becoming a mainstay for researchers working on synthetic schemes. Chemists hunting for new ways to alter molecular frameworks or assemble complex drug candidates inevitably run across this compound because of its reliable structure and versatile nature. Its commercial relevance grew as demand increased for well-identified, pure chemicals tailored for high-throughput screening and molecular design.
4-Morpholinopiperidine weighs in at 172.27 g/mol. The melting point usually hovers between 47°C and 51°C, and it comes with a modest boiling point. With its two basic nitrogens, the molecule dissolves quickly in polar solvents, especially water and alcohols, but it's less friendly to non-polar liquids. The presence of both a secondary amine and a morpholine oxygen creates reactivity patterns that synthetic chemists keep coming back to, offering multiple sites for substitution or protection. Its solid, stable form means it handles shipping and long-term storage without much fuss, as long as it's kept dry and away from oxidizers.
On the label, buyers expect to see 4-Morpholinopiperidine listed by name, CAS number 5570-77-4, molecular formula C9H18N2O, batch number, production date, and purity often above 98%. Analytical certificates show spectroscopy profiles, usually NMR and mass spectrometry, to prove quality. Handling instructions outline moisture and oxidation sensitivity, and disposal considerations reference nitrogenous organic waste. Safety data sheets sit alongside, mapping out inhalation, ingestion, and skin contact risks.
Most labs build 4-Morpholinopiperidine through cyclization reactions that start with piperidine and morpholine derivatives, bringing the rings together using an alkylation or condensation strategy, often with strong base and tailored solvents. Some processes focus on nucleophilic substitution, attaching a morpholine group to a pre-activated piperidine. Yields vary based on control of temperature and careful addition of reagents, under nitrogen to keep things dry and oxygen-free. Purification takes multiple recrystallization or column chromatography passes, especially to meet the pharmaceutical industry's high demand for purity. Bench chemists rely on clear protocols—not just to make the product, but to make sure impurities don’t sneak into research or production streams.
4-Morpholinopiperidine's structure presents plenty of opportunities to push synthesis forward in new ways. Its secondary amine nitrogen acts as both a nucleophile and a coordination site, so it takes on electrophilic substituents without much coaxing, allowing for N-alkylation and acylation. The morpholine oxygen opens a door for further modification; chemists sometimes carry out oxidations or substitutions on this position to tailor physical or biological activity in downstream products. Ring expansion, contraction, or even opening the rings under strong acidic or basic conditions provides entry to whole new classes of molecules, extending its value as a chemical platform.
Chemists and suppliers refer to 4-Morpholinopiperidine by several names. "1-(Piperidin-4-yl)morpholine" describes its connectivity. Other close analogs and trade names might pop up in pharmaceuticals or catalogs, always flagging the combined presence of the morpholine and piperidine rings. To avoid confusion, researchers stick to CAS numbers or IUPAC names when communicating across labs or ordering supplies.
4-Morpholinopiperidine doesn't wave big red flags on toxicity, but like all amine-containing compounds, it can irritate the eyes, skin, and respiratory tract. Gloves, goggles, and fume hoods make up standard protection. In my own bench work, I've seen spills and splashes, and quick cleanup with absorbents plus disposal in marked nitrogenous waste streams keeps workspaces safe. Large-scale operations focus not only on exposure but also on containment and air scrubbing, especially since amine vapors can build up in enclosed environments. Emergency eyewash stations and clear procedures for incidental contact aren't luxuries—they're part of any reputable lab's routine.
Pharmaceutical research soaks up most of the global output for 4-Morpholinopiperidine. It serves as a scaffold in the design of inhibitors targeting CNS disorders, oncology, and infectious diseases, thanks to the bioactive profile carried by piperidine-morpholine systems. Medicinal chemists see it as a jumping-off point for new molecules—antidepressants, antipsychotics, enzyme inhibitors, or as auxiliary groups to boost water solubility. Some agrochemical researchers take it up for pesticide or fungicide leads, using its nitrogen density to disrupt metabolic pathways in target species. On the analytical side, it acts as an internal standard for mass spectrometry and chromatography, proving its stability and unique signal.
4-Morpholinopiperidine sits right in the crosshairs of modern R&D. Combinatorial chemistry projects use it to populate compound libraries, seeking out new binding motifs. As more labs lean on high-throughput screening, demand rises for building blocks that support both diversity and selectivity. Researchers probe its reactivity not just for direct drug design but also as a handle for bioconjugation and molecular imaging. Academic publications track new modification protocols, green chemistry optimizations, and asymmetric synthesis routes—a sign of the relentless push for efficiency and sustainability in chemical manufacturing. Collaboration between universities, startups, and pharmaceutical giants relies on this kind of trusted, versatile intermediate to bridge the gap between theory and manufacture.
Toxicologists have checked the main boxes for 4-Morpholinopiperidine: acute toxicity sits in the moderate range, with no strong evidence for carcinogenicity or mutagenicity when handled at lab scales. Chronic exposure data remains thin, so most institutions err on the side of caution, minimizing contact and keeping logs of usage. Animal studies for structurally related piperidine and morpholine derivatives help fill in the blanks, flagging risk when pushed to high doses, especially affecting liver and kidney function. Responsible research includes environmental monitoring since nitrogen-rich organics sometimes persist in waterways. Waste treatment plans strive to break down amine-bearing molecules fully before discharge.
As drug discovery moves toward modularity, chemical frameworks like 4-Morpholinopiperidine step into the spotlight. Demand isn't going anywhere; more fields—gene modification, nanochemistry, advanced material synthesis—look for reliable, nitrogen-rich building blocks. Upcoming challenges put pressure on supply chains to deliver greener, scalable production routes. Computational chemists plug its structure into AI-driven molecule generators, looking for the next leap forward. My sense, drawn from project meetings and literature searches, points to a compound with staying power—a keystone for creative synthesis and problem-solving in life sciences and materials research. With the regulatory bar rising and the push for safer, more efficient laboratories growing louder, 4-Morpholinopiperidine has the chance to evolve with the needs of tomorrow's chemistry.
Walk into any modern lab that tracks drug discovery, and you’re likely to see vials labeled with curious names. 4-Morpholinopiperidine is one of them. It’s a mouthful, but this compound plays a big role, mostly under the hood of pharmaceutical research. I’ve had my own brushes with academic chemistry labs. Watching grad students sketch out complex structures on whiteboards, it’s easy to underestimate how much depends on the right building blocks. 4-Morpholinopiperidine stands out as one of those blocks that helps tweak bigger molecules—usually in small quantities—during drug design. Pharmaceutical chemists rely on it for its ability to add a specific shape and flexibility to molecules under study.
Pharmaceutical pipelines can eat up millions of dollars and years of labor before anything useful hits a hospital shelf. 4-Morpholinopiperidine hasn’t earned a starring role as a medicine itself, but its true impact shows up behind the scenes. Synthesis teams use it to help assemble new chemical entities, the little scaffolds that act as blueprints for drugs. Whenever researchers aim to modulate the activity of a molecule—maybe to make an antidepressant work better or reduce a side effect—they might turn to chemical fragments like this one. In practice, compounds built with its backbone have appeared in the literature for years, and research keeps turning up novel uses.
Chemicals like this don’t circulate freely. There’s a flip side to the power they bring: misuse risk. Stories about precursor chemicals and illicit manufacturing aren’t uncommon. That’s one reason regulatory eyes stay fixed on compounds like 4-Morpholinopiperidine. In many places, purchase and possession ask for solid paperwork and purpose. This protects patients and researchers alike from accidents, contamination, or diversion into black markets. Growing up surrounded by emergency services folks, I saw firsthand that it’s not some distant threat—lax handling can result in real, community-level harm.
The business of drug development leans hard on chemicals like this. Startups and big-pharma both need access, but public trust depends on how industry stewards that access. With recent focus on ethical sourcing, waste management, and data sharing, there’s rising pressure not only to discover quickly but to avoid corners that lead to environmental or human risk. The vast majority using 4-Morpholinopiperidine just want to make better medicines. Still, it only takes one bad apple misusing a precursor to disrupt supply chains and prompt tighter restrictions for everyone.
Instead of taking for granted these supporting players, we ought to keep asking: are there greener, safer alternatives? Green chemistry movements look for substitutes that leave less environmental residue and aren’t liable to misuse. Training new lab workers in chemical stewardship matters just as much. Tools like 4-Morpholinopiperidine make groundbreaking drugs possible, but only in the right hands. Its value doesn’t come from the name or even the molecule itself—it comes from tackling big problems like pain, infection, or neurological disease, with every bottle tightly managed and responsibly used along the way.
4-Morpholinopiperidine pops up in some chemistry labs as a building block for pharmaceutical and research applications. This stuff looks harmless on paper, but experience warns me that anything with that kind of structure commands a double-take when it appears on a chemical list for a project. It’s neither a household item nor something to treat casually. Even seasoned scientists pause before unscrewing a bottle like this.
The Material Safety Data Sheet for 4-Morpholinopiperidine rarely sugar-coats the hazards. Breathing the vapors or skin contact can cause harm. Laboratory protocols flag this one as harmful through inhalation, skin, or eye contact. Toxicity isn’t at the extreme end, yet it sits in a range where a spill, splash, or breathing in the fumes can lead to irritation, headache, or worse. Many chemists share stories of stinging skin or red eyes after an unexpected exposure, sometimes even under a fume hood. This isn’t me telling scary campfire stories—it’s lab reality, plain and simple.
It’s easy to shrug off chemical safety, especially when you’ve worked in a lab for years. That attitude can change after a single sloppy moment with something like 4-Morpholinopiperidine. People get used to cutting corners. Maybe gloves seem a hassle. Old goggles fog up, so somebody leaves them behind without thinking. Then a splash or a drop in the wrong place reminds everyone why those rules exist. In my own early days, I learned to respect every warning label after one hasty experiment with a similar reagent left my hands tingling for hours. You only need one bad day to understand the value of a careful setup.
For chemicals like 4-Morpholinopiperidine, wearing the right gloves, lab coat, and goggles isn’t an extra step—it’s the only smart way to work. But personal protective equipment isn’t enough without solid ventilation. A good fume hood takes most of the risk off the table. Still, even the best hoods won’t help if you lean in with your hands bare or let a bottle sit open too long. Most experienced chemists keep a mental checklist: lower the sash, check the exhaust, suit up, and seal everything tight after use.
It’s not about avoiding 4-Morpholinopiperidine entirely. The answer lies in training lab crews to spot risks before they turn into trouble. Regular refreshers about chemical hazards work better than a pile of unread binders. I’ve seen teams share quick “near miss” stories and review them at the start of each month—that habit keeps people sharp. Simple changes like color-coded storage or mandatory glove changes between steps cut down on accidental exposure. If a lab shows spills or sloppy areas, that problem rarely stays confined to one chemical.
Working with chemicals like 4-Morpholinopiperidine demands a mindset that favors caution over speed. No experiment or deadline justifies ignoring safe handling. Hearing stories from colleagues who faced burns or breathing issues continues to reinforce the message: use common sense, keep up the right habits, and don’t let familiarity cause carelessness. Safety culture grows stronger with each lesson learned and shared.
The chemical world is full of strange names, and 4-Morpholinopiperidine is one that looks complicated at first glance. In practice, it’s no monster—just the fusion of two interesting rings: piperidine and morpholine. Imagine a hexagon from piperidine where one carbon has been replaced by a nitrogen to make a six-membered ring, and then imagine a four-membered morpholine ring whose oxygen and nitrogen give it unique properties. When chemists talk about 4-Morpholinopiperidine, they’re describing a compound where morpholine hangs off the fourth carbon atom of a piperidine ring.
Here’s what really makes it tick: 4-Morpholinopiperidine links two ring systems together. The six-membered piperidine ring does some heavy lifting in pharmaceuticals thanks to its flexibility and tendency not to break down easily in water. The morpholine ring, meanwhile, adds polarity with its oxygen. This combination turns out to be popular with medicinal chemists because it tunes the balance between water-solubility and molecular stability. By merging these two rings, the molecule achieves a rare mix of rigidity, versatility, and resistance to metabolic breakdown.
Piperidine-based compounds show up in medicines all the time. If you’ve ever seen antihistamines or antidepressants, you’ve likely encountered molecules shaped like piperidine. Morpholine derivatives show up in certain anticancer drugs and in the world of organic synthesis as handy building blocks. 4-Morpholinopiperidine stands out due to its ability to combine the best traits of both ring systems, making it useful in drug design. When researchers adjust drug properties to increase their effectiveness in the body, they often need molecules that provide just the right stability and interaction with proteins. 4-Morpholinopiperidine can help with both tasks because of its balanced design.
Working with 4-Morpholinopiperidine isn’t as easy as mixing salt and water. Chemical synthesis requires stable building blocks and precise control over reaction conditions. For chemists in the lab, handling nitrogen-containing compounds always means paying extra attention to purity and safety. Impurities can lead to side reactions that create unwanted byproducts, raising concerns about both yield and safety. This is a recurring challenge in pharmaceutical manufacturing. The drive to improve purification and streamline the synthetic steps isn’t just about saving money, but also about protecting researchers and ensuring public confidence in the medicines we use.
The story of 4-Morpholinopiperidine demonstrates the importance of robust chemical knowledge. Its design highlights how a small tweak in ring attachment can transform the uses of a molecule. Organizations working with such chemicals benefit from transparent research, careful safety practices, and open discussion of challenges. Research into analogs of this molecule follows best practices by public disclosure, peer review, and safety-first laboratory protocols. These values align with the current push for trust in research and evidence-based advances in health and technology. Keeping strong training programs and ethical controls protects both the research community and the broader public.
4-Morpholinopiperidine—sounds technical, but it’s just one of many chemicals making the wheels turn in labs and industry. Open up any supply cabinet and the old rules come straight to mind: safety first, shortcuts second. I remember walking into a lab as a rookie and seeing labels faded and bottles shoved together. That’s not the place to stick a multipurpose tool like piperidine derivatives.
Ask anyone who’s worked with organic compounds for a few years, and they’ll right away point to temperature and humidity as troublemakers. 4-Morpholinopiperidine likes a cool, dry place. Storing it away from direct sunlight isn’t just about avoiding a mess—it protects potency and helps avoid dangerous reactions. Many seasoned chemists reach for temperature logs, and digital thermometers fastened to the fridge door are a common sight. My own experience taught me that chemicals left on a benchtop become unreliable fast, not to mention the risk of contamination.
Many organic compounds, including this one, pull water out of the air or break down if left uncapped. I’ve seen bottles puff up and strange smells creep out. Screw caps and crimped seals save both money and safety headaches. Flammable safety cabinets offer an extra layer of protection, and they aren’t just for show; anyone familiar with surprise compliance checks knows an inspector will open every bright yellow cabinet, looking for slips. Glass or compatible HDPE bottles with tight-fitting lids work. Stained glass isn’t just for art—it blocks out UV and keeps the light from doing weird things to chemicals.
Clear labels are a cornerstone of any responsible lab. No one wants to play the guessing game with unidentified bottles. I’ve seen close calls traced back to missing or peeling labels. Use permanent marker, slap on a clear sticker, and don’t rely on memory. Details matter: write the full name (not the shorthand only your supervisor uses), the date it arrived, and the name of whoever shelved it. Swapping stories with others, most agree accidents tend to happen less often when storage areas are tidy and marked.
Chemicals have bad neighbors. Acids, oxidizers, and strong bases need their own neighborhoods—never lump them into the same box as amines or proprietary intermediates. I learned early on that fire and chemical spill reports rarely come from well-organized shelves. Since 4-Morpholinopiperidine has both an amine and a piperidine ring, it should stay far from oxidizers and acids. Reading material safety data sheets (SDS) isn’t a formality—it’s a roadmap to a safer lab.
Rotating stock keeps everything fresh. I pull expired bottles off the shelf and check for leaks or weird color changes at least every quarter. It’s not just a formality for audits; it stops incidents before they start. If something smells off or looks strange, proper disposal beats gambling with safety. Local waste regulations tell you what to do next, and there’s no shame in calling hazardous waste services for help.
Many safety drills skip over the simple stuff. Using posters, exception logs, or even phone photos of the correct storage setup—these reminders cut down on lazy habits. From old hands to newcomers, clear habits protect health, time, and reputation.
4-Morpholinopiperidine stores safest in a system that follows well-worn habits: cool air, dry shelf space, dark corners, airtight lids, and labels that won’t fade. Trusted procedures mean fewer surprises, for yourself and the next person who opens that cabinet.
Looking up 4-Morpholinopiperidine usually means stepping into the world of complex research chemicals. This compound doesn’t cater to hobbyists or casual buyers. If you need it, you’re likely working in a legitimate lab, developing new pharmaceuticals, or running important experiments. Most of the time, my experience says researchers source such chemicals through verified suppliers who demand strict records and clear reasoning behind every purchase. It keeps everyone honest and public safety front of mind.
Lawmakers and enforcement agencies see 4-Morpholinopiperidine as sensitive stuff, not just another ingredient. This compound can serve as a building block for other chemicals, sometimes including restricted pharmaceuticals. That’s why most chemical suppliers perform background checks, require end-use statements, and only ship to licensed organizations. Some marketplaces even cross-check buyers with regulatory lists. I’ve found that nobody sells you a bottle just because you click “add to cart.”
Reputation holds real weight in the chemical trade. Suppliers like Sigma-Aldrich, Alfa Aesar, TCI America, and Acros Organics provide 4-Morpholinopiperidine to researchers who can prove professional intent. These companies expect proper documentation, such as business licenses and lab accreditation. Purchasers often supply a thorough explanation of how and why the chemical is being used. There’s a responsibility to keep controlled substances out of the wrong hands, and these companies take it seriously for good reason. I’ve worked with suppliers like these and every interaction runs on trust and transparency.
Attempting to bypass legal controls leads to considerable risks. Law enforcement has cracked down hard on so-called “grey market” sellers or those advertising chemicals under slightly altered names. Besides running serious legal risks, buying from these sources may leave you with impure or mislabeled products, putting your research—and your licenses—on the line. One bad batch can ruin months of careful work, or far worse, harm lives. That risk simply isn’t worth taking.
Colleagues who handle tricky compounds focus on building strong relationships with reputable suppliers, staying educated about current regulations, and keeping documentation up to date. If your lab needs something like 4-Morpholinopiperidine, talk directly to manufacturer representatives or regional distributors. Some countries have unique regulations, so check both local and international rules before any purchase. Most important: maintain clear records of every transaction and use.
Handling regulated chemicals brings a real responsibility. That’s true whether you’re a scientist working on a new medicine or overseeing a quality control process. Buying from established sources protects not just the research, but also your reputation and the safety of everyone who might handle the compound after you.
| Names | |
| Preferred IUPAC name | 4-Morpholin-4-ylpiperidine |
| Other names |
4-(Morpholin-4-yl)piperidine N-(4-Piperidinyl)morpholine |
| Pronunciation | /fɔːr-mɔːrˌfəˈliːnoʊ-paɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 22203-74-1 |
| Beilstein Reference | 613849 |
| ChEBI | CHEBI:78166 |
| ChEMBL | CHEMBL140639 |
| ChemSpider | 83580 |
| DrugBank | DB08797 |
| ECHA InfoCard | 03db640c-86aa-44d7-81bc-d717b2f4167a |
| EC Number | EC 606-448-8 |
| Gmelin Reference | 78753 |
| KEGG | C21103 |
| MeSH | D039523 |
| PubChem CID | 180753 |
| RTECS number | TI1575000 |
| UNII | 662A9T2QG8 |
| UN number | UN3431 |
| Properties | |
| Chemical formula | C9H18N2O |
| Molar mass | 172.27 g/mol |
| Appearance | Colorless to yellowish liquid |
| Odor | Amine-like |
| Density | 1.05 g/mL at 25 °C(lit.) |
| Solubility in water | Soluble |
| log P | -0.16 |
| Vapor pressure | 0.4 mmHg (25 °C) |
| Acidity (pKa) | 9.64 |
| Basicity (pKb) | pKb = 3.79 |
| Magnetic susceptibility (χ) | -78.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.528 |
| Viscosity | 0.991 cP |
| Dipole moment | 3.16 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 329.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06 |
| Signal word | Warning |
| Hazard statements | H302, H312, H332 |
| Precautionary statements | P264, P270, P261, P271, P280, P301+P312, P330, P304+P340, P312, P405, P501 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 94 °C |
| Autoignition temperature | 235 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 485 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50 465 mg/kg |
| NIOSH | FH2100000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 4-Morpholinopiperidine is not specifically established by OSHA or NIOSH. |
| REL (Recommended) | Intermediate |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Morpholine Piperidine N-Methylmorpholine N-Phenylpiperidine 4-Piperidone N-Boc-piperidine N-Methylpiperidine |